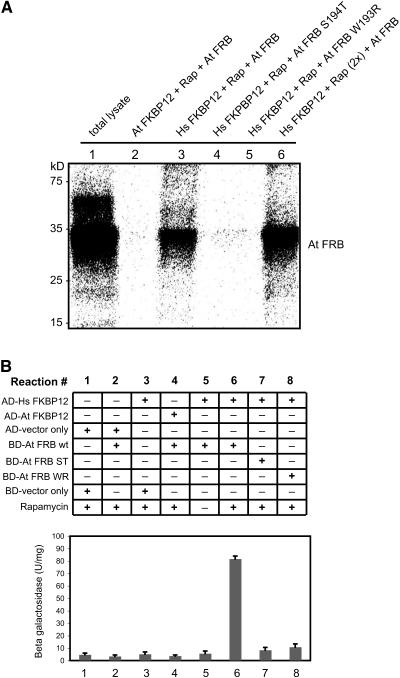
Figure 9.
Rapamycin-Dependent Interaction between TOR-FRB Domain and Hs FKBP12.
(A) In vitro assay for TOR-FKBP12 interactions. The [35S]Met-labeled TOR-FRB domain and its mutant variants (At FRB S194T and At FRB W193R) were prepared by in vitro transcription/translation system. GST-fusion proteins of At FKBP12 and Hs FKBP12 were expressed in E. coli and purified. All binding assays except for lane 1 (total in vitro translation lysate) included rapamycin (Rap). The amount of rapamycin was doubled in lane 6. The binding assay samples were pulled down with GST-Sepharose and loaded onto SDS-PAGE followed by autoradiography. Hs FKBP12 showed an interaction with At FRB domain in the presence of rapamycin (lanes 3 and 6), while At FKBP12 or the mutant form of Hs FKBP12 failed to form a complex with At FRB (lanes 2, 4, and 5).
(B) Yeast two-hybrid assay showing the TOR-FKBP12 interaction in vivo. GAL-4 (BD) fusion construct of TOR-FRB wild type and mutants W193R (WR) and S194T (ST) were coexpressed with GAL-4 (AD) fusion constructs of At FKBP12 and Hs FKBP12 in the yeast strain SMY4 as indicated in the figure. β-Galactosidase assays were performed in liquid culture using o-nitrophenyl-d-galactopyranoside as a substrate as described in Methods. The results are represented as the average of four independent experiments ± se.