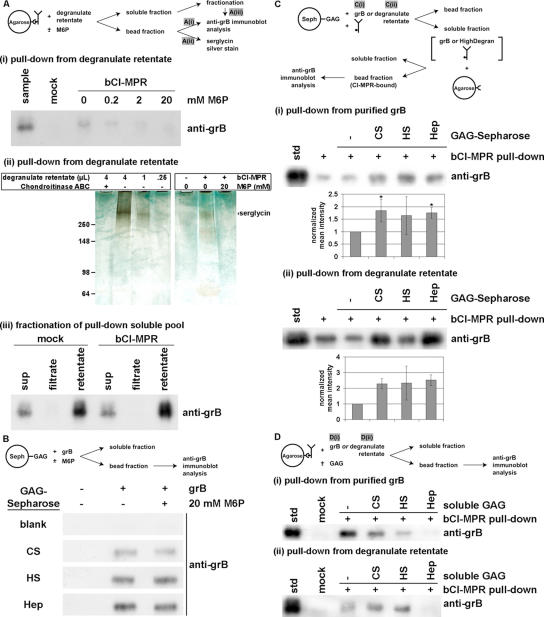
Figure 4.
Granzyme B binding to CI-MPR is inhibited by mannose 6-phosphate and soluble heparin, but is enhanced by immobilized GAGs. (A) To obtain a concentrated source of predominantly serglycin-complexed grB, CTL degranulate material was obtained as the supernatant of a CTL culture and incubated for 4 h at 37°C in the presence of immobilized anti-CD3 antibody. Subsequently the degranulate was fractionated over a 100-kDa cutoff filter, and the high molecular retentate was utilized. Degranulate retentate was incubated with biotinylated, soluble CI-MPR (bCI-MPR) prebound to Neutravidin-Agarose beads. In some samples M6P (0.2–20 mM) was present during incubation. (i) The bead fraction was probed by immunoblot to detect grB. (ii) The bead fraction was resolved on a 4–15% gradient SDS-PAGE, and sensitivity-enhanced silver staining was performed to detect proteoglycans. As a control to identify serglycin, degranulate retentate was treated with or without chondroitinase ABC, and was analyzed in titrated amounts. (iii) The soluble fraction of samples was recovered and fractionated over a 100-kDa cutoff filter. Equal volumes of samples were analyzed by immunoblot to detect grB. (B) Purified grB was incubated with GAG-Sepharose beads (CS, chondroitin sulfate A; HS, heparan sulfate; Hep, heparin), in the presence (+) or absence (-) of 20 mM M6P. The bead fraction was probed by immunoblot to detect grB. (C) Purified grB (i) or degranulate retentate (ii) was incubated with free bCI-MPR in the presence or absence of GAG-Sepharose beads (total GAG estimated at 10-fold molar excess of grB). The soluble fraction was then applied to Neutravidin-Agarose beads in order to immobilize bCI-MPR. Subsequently, the bead fraction was probed by immunoblot to detect grB. Band intensity was quantified by densitometry and is expressed as the normalized mean ± SD (i; n = 3) or ± range (ii; n = 2) (* 0.01 < p < 0.05). (D) Purified grB (i) or degranulate retentate (ii) was incubated with bCI-MPR prebound to Neutravidin-Agarose beads. In the indicated samples, GAG was present at 500-fold molar excess of grB. The bead fraction was probed by immunoblot to detect grB. Notably, chondroitin sulfate and heparan sulfate had inconsistent effects on grB binding to the bCI-MPR, therefore cannot be interpreted reliably. On the other hand, consistently heparin effectively blocked grB binding to bCI-MPR; when band intensity was quantified by densitometry, compared with the std in the absence of GAG (normalized to 1.0), heparin diminished grB recovery to (i) 0.23 ± 0.15 (0.001 < p < 0.01; n = 3) and (ii) 0.26 ± 0.21 (0.01 < p < 0.05; n = 3).