Figure 9.
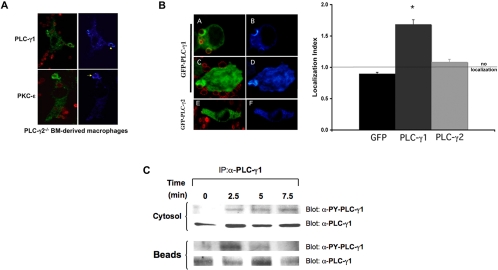
PLC-γ1 is involved in IgG-mediated phagocytosis. (A) Endogenous PLC-γ1 and protein kinase C-ε concentrate at phagosomes in PLC-γ2-/- BMDMs. PLC-γ2-/- BMDMs were incubated with BIgG targets (2.5–10 min), fixed and stained for PLC-γ2 (top) or protein kinase C-ε (bottom). Left column, merge image Alexa 488 (PLC-γ1protein kinase C-ε) and Alexa 568 (BIgG); right column, pseudocolor for Alexa 488. Representative of three experiments with similar results. (B) PLC-γ1 but not PLC-γ2 accumulates at forming phagosomes. RAW cells were transiently transfected with GFP-PLC-γ1 (A–D) or GFP-PLC-γ2 (E and F). Phagocytosis of BIgG was terminated at 5–7.5 min. Cells were fixed, and GFP distribution was detected by confocal microscopy. The localization index for GFP, GFP-PLC-γ1, or GFP-PLC-γ2 was calculated (>30 events from 3 independent experiments for each construct). Data are presented as mean ± SEM, *p < 0.0004 compared with GFP. (C) PLC-γ1 is phosphorylated during IgG phagocytosis. Bead-associated nascent phagosomes were separated from the nonbead cytosolic fraction at the indicated times during synchronized phagocytosis. Both fractions were solubilized and immunoprecipitated with antibody to PLC-γ1. The associated proteins were separated by SDS-PAGE and blotted for active PLC-γ1 using the activation-specific anti-PLC-γ1 antibody (recognizes phospho-tyrosine 783 on PLC-γ1) and PLC-γ1. Representative of three similar experiments.