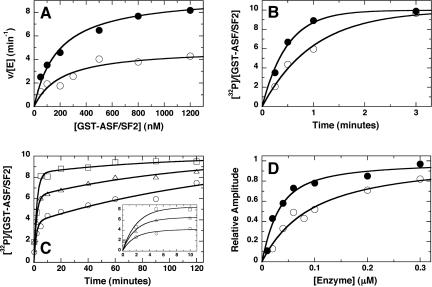
Figure 4.
Effects of the SRPK1 spacer on the phosphorylation kinetics of ASF/SF2. Steady-state kinetics (A). Initial velocities were measured as a function of varying GST-ASF/SF2 using SRPK1 (•) and K1-ΔS (○). For SRPK1, kcat and Km are 9.5 ± 0.5 min-1 and 190 ± 40 nM, respectively, and for K1-ΔS, kcat and Km are 4.9 ± 0.4 min-1 210 ± 60 nM, respectively. and Single-turnover kinetics (B). GST-ASF/SF2 (200 nM) was preequilibrated with 1 μM SRPK1 (•) and K1-ΔS (○) and allowed to react with [32P]ATP for varying periods of time. Data were fit to a single exponential function to obtain rate constants of 2.5 and 0.9 min-1 for SRPK1 and K1-ΔS. Enzyme-dependent single turnover kinetics (C). GST-ASF/SF2 (10 nM), preequilibrated with 50 nM (○), 150 nM (▵), and 360 nM (□) K1-ΔS, was mixed with [γ-32P]ATP for varying periods of time, and the number of sites phosphorylated was determined and expressed as a ratio of32P-incorporated and the total GST-ASF/SF2 concentration. The amplitudes of the first kinetic phase are 3.7, 6.2, and 8 at 50, 150, and 360 nM K1-ΔS, respectively. Determination ofKd values for SRPK1 and K1-ΔS (D). The amplitudes of the first phases in single turnover experiments were normalized to the reaction endpoints and plotted against the total concentrations of SRPK1 (•) and K1-ΔS(○). A quadratic function was used to obtain K values of 20 ± 6 and 80 ± 14 nM for SRPK1 and K1-ΔS, respectively.d