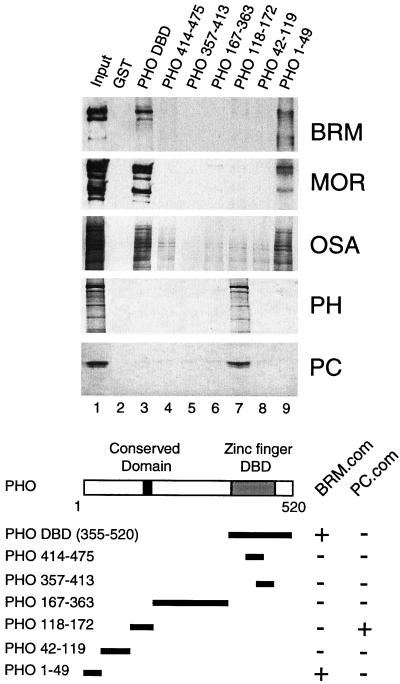
FIG. 2.
PHO interacts with PcG proteins and the BRM complex. The ability of various PHO polypeptides to recruit PC, PH, and the BRM complex from Drosophila embryo nuclear extracts was tested by GST pulldown assays. GST alone (lane 2), GST-PHO DBD (amino acids 355 to 520; lane 3), GST-PHO(414-475) (lane 4), GST-PHO(357-413) (lane 5), GST-PHO(167-363) (lane 6), GST-PHO(118-172) (lane 7), GST-PHO(42-119) (lane 8), and GST-PHO(1-49) (lane 9) were immobilized on glutathione-Sepharose beads and incubated with a partially purified column fraction containing the PC and BRM complexes. Protein complexes were washed, resolved by SDS-PAGE, and transferred to nitrocellulose. The blots were probed with antibodies directed against BRM (39), MOR (SN670 and SN671, pooled), OSA (74), PH (PV86), or PC (PV69). Lane 1 represents 5% of the input material used in the binding reactions. The domain structure of PHO, including the zinc finger DNA-binding domain (DBD), a conserved region present in YY1, and the amino acid residues present in the various derivatives are indicated. The binding of the PHO deletion constructs to either the BRM complex (BRM.com) or the PC complex (PC.com) is summarized.