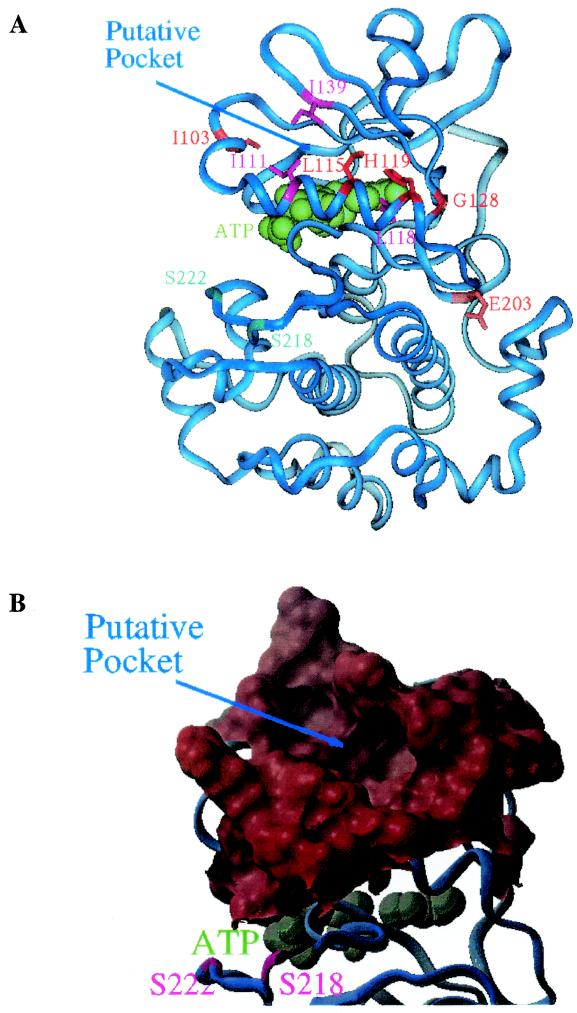
FIG.5.
MEK homology model illustrating PD 184352 interacting mutations and putative binding pocket. (A) Ribbon diagram of a MEK1 homology model. The molecule is viewed from the rear side of the ATP binding pocket with the N-terminal lobe in the upper region and the C-terminal lobe in the lower region. Residues that were identified to perturb activity of PD 184352 are shown in sticks and colored red or magenta. Residues I103, L115, H119, G128, and E203 (red) were identified by random screening, whereas residues I111, I118, and I139 (magenta) were proposed based on the homology model. ATP is colored green and is shown in space-filling mode. Serine residues S218 and S222, which are phosphorylated when MEK1 is activated in vivo, are colored cyan. (B) Surface diagram N-terminal lobe showing the putative binding pocket. This diagram is viewed in the same orientation as for panel A. ATP is colored blue-green and is shown in space-filling mode.