Abstract
Background and Aim:
Budd-Chiari syndrome (BCS) is a rare condition associated with hepatic venous outflow obstruction classically treated with portosystemic shunts or liver transplantation. Recent reports indicate promising results with the use of transjugular intrahepatic portosystemic shunts (TIPS) in the treatment of these patients.
Patients and Methods:
We reviewed a 10-year single-institution experience with TIPS in patients diagnosed with BCS.
Results:
Eleven patients with BCS underwent TIPS procedures, 3 of whom carried a diagnosis of paroxysmal nocturnal hemoglobinuria, a relative contraindication for liver transplantation. One TIPS procedure was unsuccessful for technical reasons. No patient suffered mortality or major morbidity related to the TIPS procedure. The mean reduction of portal venous pressures was 43.7%, with a mean decrease of 73% in the pressure gradient. Of the 7 patients where long-term follow-up was available, 57% had shunts which remained patent but required several nonsurgical revisions for occlusion, with an average assisted patency of 37.5 months.
Conclusions:
TIPS is an effective modality in the treatment of patients with BCS, especially for those who are not candidates for liver transplantation. TIPS can be successfully used as a bridge to surgical portosystemic shunting, as well as liver transplantation, but may cause technical difficulties when performing transplantation.
TIPS is an effective modality in the treatment of patients with BCS, especially for those who are not candidates for liver transplantation. TIPS can be successfully used as a bridge to surgical portosystemic shunting as well as liver transplantation, but may cause technical difficulties when performing transplantation.
Hepatic venous outflow obstruction, also known as Budd-Chiari syndrome (BCS), is a rare condition that causes hepatic congestion, portal hypertension, hepatocyte necrosis, and eventual liver failure. In the form most commonly encountered in the United States, BCS is caused by thrombotic occlusion of the hepatic veins or inferior vena cava (IVC), and can usually be attributed to several thrombogenic disorders, most commonly myeloproliferative diseases.1,2 Clinical manifestations include abdominal pain, liver dysfunction, and intractable ascites. Ascites is the most common clinical feature of BCS and is also the most frequent reason for referral for nonmedical therapy.3–5
Surgical options are primarily limited to mesenteric-systemic shunts and liver transplantation.6–9 The therapeutic decision should be based on liver histology, pressure gradients, and clinical factors. Furthermore, premalignant conditions, such as paroxysmal nocturnal hemoglobinuria (PNH),10,11 are relative contraindications for liver transplantation. Both treatment modalities were recently reviewed in our institution with good resolution of symptoms and 5-year survival rates in excess of 75%.12
The use of transjugular intrahepatic portosystemic shunts (TIPS) in the treatment of cirrhosis and portal hypertension has been reviewed in many series.13,14 Standard indications include acute variceal bleeding and ascites refractory to paracentesis. An important factor limiting the success of TIPS is shunt stenosis.14 This necessitates continued surveillance for patency and often multiple interventions for continued shunt function.15
The utility of TIPS in the treatment of BCS has been reviewed in several small series and case reports with promising results.16–21 Similar problems associated with repeated shunt occlusion seem to occur in patients with BCS. The purpose of our study was to evaluate the etiology, clinical aspects, and outcome of BCS patients treated with TIPS in our institution, with the ultimate goal of better elucidating the role of this nonsurgical procedure in the treatment of BCS.
EXPERIMENTAL PROCEDURES
TIPS procedures were performed on 219 patients between January 1993 and January 2003. Eleven of the 219 (5%) were diagnosed with BCS based on clinical findings, color Doppler sonography, venography findings at the time of TIPS procedure, and other imaging studies including computed tomography and magnetic resonance imaging. The TIPS procedure was performed in the usual fashion as previously described.12,22 An internal jugular vein approach was used in all patients using a standard 11-French sheath. Cannulation of the right or middle hepatic veins was initially attempted in all patients using a selective multipurpose catheter (Cordis, Miami FL). In patients in whom the hepatic veins were not visualized during venography, the TIPS procedure was performed from the intrahepatic IVC to the left portal vein. Access into the portal vein was achieved with a Colopinto needle (Cook, Bloomington, IN) that was advanced through the liver parenchyma into the portal vein using standard fluoroscopic landmarks. Through the needle system, a Wholey wire (Boston Scientific, Natick, MA) was advanced into the mesoportal venous system followed by the placement of a standard pigtail catheter (Cook). Portal pressures were obtained followed by a portogram to evaluate the portal anatomy. Next, the tract was dilated with a 6-mm angioplasty balloon (Cordis) over a standard stiff wire. The TIPS was created through the insertion of a Wallstent (Boston Scientific, Natick, MA) that was 8 to 12 mm in diameter that extended from the main branch of the portal vein into the IVC (standard lengths, 5–7 cm).
Poststent portograms and pressure measurements were performed to assess the degree of decompression of the portal venous system. Follow-up included inpatient evaluation and visits to the outpatient clinics in our hospital. During the first year, we routinely performed serial ultrasonographic evaluations. Thereafter, we recommended such evaluations on a semi-yearly or yearly basis. Our experience and previous studies showed as well that clinical examination was also a sensitive way to detect TIPS occlusions, since patients almost always presented with recurrent ascites. Although at the beginning of our experience we only anticoagulated those patients with BCS and demonstrated thrombotic disorders, currently we place all patients with BCS on anticoagulation regimens. Several patients were lost to follow-up as indicated.
RESULTS
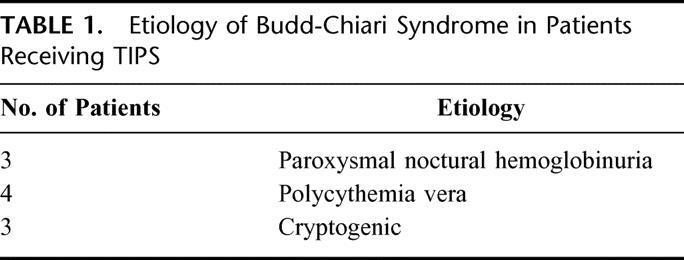
Eleven patients were referred for TIPS for BCS with intractable ascites. Of these patients, 6 were female and 5 male. Two patients had undergone mesocaval shunt placement prior to referral for TIPS, one of which failed after 4 years and the other one after 6 years. One patient had a stent placed in the IVC that was functional for 15 years and subsequently required TIPS. With regards to the etiology of the BCS, 3 of these patients carried a diagnosis of PNH and 4 had polycythemia rubra vera (PCV), a common myeloproliferative disorder (Table 1).
TABLE 1. Etiology of Budd-Chiari Syndrome in Patients Receiving TIPS
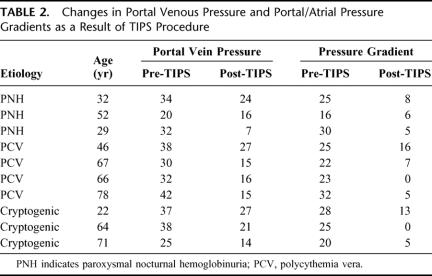
TIPS were successful in 10 of the 11 patients (91%). The one failure was due to the technical inability to cannulate the thrombosed hepatic veins or the IVC directly, and ultimately required mesocaval shunting. Of the 10 patients with successful nonoperative shunts, the mean age was 53 ± 20 years (range, 22–78 years; median, 58 years). No mortality or major morbidity was associated with the TIPS procedure, and decompression was successful by clinical parameters and venous pressure dynamics (Table 2; Fig. 1) in all patients receiving shunts.
TABLE 2. Changes in Portal Venous Pressure and Portal/Atrial Pressure Gradients as a Result of TIPS Procedure
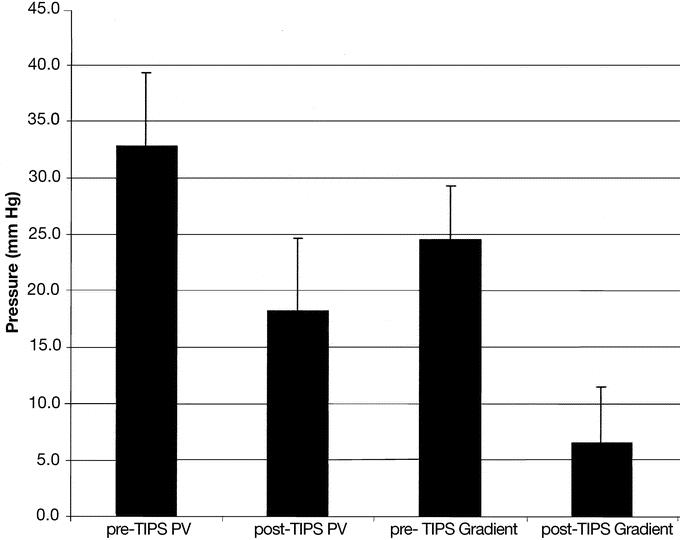
FIGURE 1. Changes in portal venous pressure (PV) and portal/atrial pressure gradients as a result of TIPS procedure.
The mean portal venous pressure dropped from a mean of 32.8 ± 6.6 mm Hg (range, 20–42 mm Hg; median, 33 mm Hg) before TIPS to a mean of 18.2 ± 6.4 mm Hg (range, 7–27 mm Hg; median, 16 mm Hg) after the procedure. This indicates an average percent reduction of portal venous pressure of 43.7% ± 18.1% (range, 20%–78%; median, 44.4%).
An even more significant reduction was seen in the portal/atrial pressure gradient, with a pre-TIPS mean gradient of 24.6 ± 4.7 mm Hg (range, 16–32 mm Hg; median, 25 mm Hg) falling to 6.5 ± 5.0 mm Hg (range, 0–16 mm Hg; median, 5 mm Hg) after TIPS. Mean percent reduction of gradient pressures was 73% ± 20% (range, 36%–100%; median, 71.6%).
Two patients returned to their referring hospitals and were lost to follow-up immediately after the procedure. One patient died after 4 months with a functioning shunt from sepsis unrelated to the TIPS procedure.
Follow-up is available for the remaining 7 patients, all of whom have required one or more revisions of their shunts. Of these, 2 patients progressed to require surgical shunts 10 and 66.5 months after TIPS procedure. Another patient, after 47 months of clinical stability with a TIPS shunt, developed progressive hepatic decompensation, underwent an orthotopic liver transplant, and died as a result of surgical complications related to this procedure. Although there were significant technical challenges during the recipient hepatectomy as a result of the prior TIPS, they were not attributable to the patient's mortality.
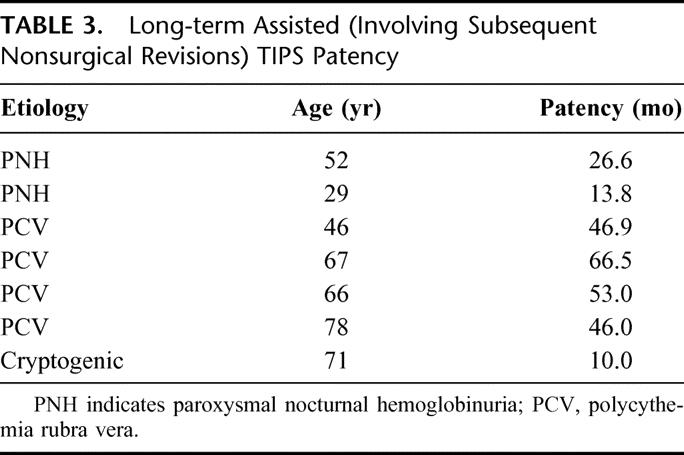
TIPS shunts remained patent (requiring occasional nonsurgical revisions) in 4 of 7 patients (57%) for whom long-term follow-up is available (Table 3), with an assisted patency of 37.5 ± 21.1 months (range, 10.0–66.5 months; median, 46.0 months).
TABLE 3. Long-term Assisted (Involving Subsequent Nonsurgical Revisions) TIPS Patency
DISCUSSION
TIPS is considered a standard treatment option for patients with portal hypertension resulting in variceal bleeding refractory to medical therapy.13 Although limitations of TIPS include shunt occlusion or dysfunction as well as hepatic encephalopathy, technical success is high and the incidence of significant morbidity or mortality as a result of the procedure itself is low.14 TIPS is considered a reasonable alternative to surgery in cirrhotic patients given the high operative risk in this patient population.
The goal of treatment of BCS is reduction of hepatic congestion and associated sequelae such as significant ascites. The etiology of BCS must be considered when planning therapeutic options. Many patients are found to have some sort of thrombophillic disorder, the most common of which is PCV. Initial management includes control of the underlying disease, control of ascites with medical therapy and paracentesis, and long-term anticoagulation. Successful treatment with anticoagulation and thrombolytic therapy alone has also been reported.2,12
Until recently, treatment of BCS refractory to medical management has been limited to surgical options, including portosystemic shunting or liver transplantation.12 These are still considered standard treatments in patients with diseases such as PCV. Patients with PNH, on the other hand, suffer from disseminated clotting, and their prognosis is considered so poor with surgery that some centers consider this disease a relative contraindication to transplantation.
Our own experience shows that, over the 2 decades from 1976 to 1996, approximately 90% of patients with BCS at our institution received surgical shunting as initial treatment. Approximately 6% had liver transplantation and approximately 4% had TIPS as their primary therapy. In the decade from 1993 to 2003, of 20 patients referred for transplant evaluation or TIPS placement, approximately 60% had TIPS first, 25% had shunt first, and 15% had liver transplantation first.
Recent studies indicate potential reduction of portal venous pressures with TIPS in the treatment of BCS. Blum et al reported successful TIPS creation in 12 of 12 patients, with average reduction of portal venous gradients by 75% and no serious complications related to the procedure itself. Half of their patients experienced subsequent shunt dysfunction requiring further interventions.16 Three of 4 patients in another study were successfully treated with TIPS,17 as were 4 of 4 in 2 other reports.18,19 Perello et al treated 13 BCS patients with TIPS. Of the 11 that survived long-term, all were free of ascites, but 8 developed shunt dysfunction.20 Finally, TIPS was technically successful in 7 of 8 patients awaiting liver transplantation in a study by Ryu et al.21 There were no reported major drawbacks to transplantation as a result of the TIPS procedure.21
Our findings corroborate the findings in the literature that patients with BCS can be successfully treated with TIPS as a bridge to transplantation, or even in lieu of surgical portosystemic shunting. Almost all patients were technically shuntable by TIPS, and those who were shunted experienced an almost 75% reduction in portal pressure gradients. We observed adequate resolution of clinical parameters and no major morbidity or mortality as a result of the procedure. Consistent with previous concerns regarding transplantation following shunts, the one patient in our series who progressed to liver transplantation was noted to have some technical challenges. Such difficulties include the migration of the TIPS proximally into the suprahepatic IVC or right atrium. These problems, however, do not overshadow those encountered at the time of transplantation after surgical portosystemic shunting.
We routinely recommend liver transplantation in BCS patients with no contraindications to the procedure that present with acute liver failure or with histologic evidence of fibrosis or cirrhosis. We view shunting as a therapy with proven long-term results for patients who do not qualify for liver transplantation, with no acute liver failure, or who show no histologic evidence of cirrhosis or fibrosis. We view TIPS as an acute decompressive bridging procedure prior to liver transplantation or surgical shunting in patients with BCS, as well as a treatment comparable to surgical shunting for which long-term results are not yet available.
CONCLUSION
Based on our data and a review of the literature, we conclude that TIPS is a safe and effective modality in the treatment of BCS, especially for patients with high surgical risk, with PNH, and nontransplant candidates. Because no prospective studies exist comparing TIPS to surgical shunt or liver transplantation, the therapeutic choices in the management of this BCS are still based on the clinical scenario and institutional experience.
Discussions
Dr. Gazi B. Zibari (Shreveport, Louisiana): This is a very well-written manuscript addressing Budd-Chiari syndrome, which is a very challenging medical condition surgeons often have to address when medical management fails.
The authors have reviewed a 10-year Hopkins experience with TIPS procedures. They performed approximately 219 TIPS procedures. Eleven of these were done for Budd-Chiari syndrome, roughly averaging 1 patient per year with over 90% success rate, zero mortality, no major complications, and they observed good clinical response.
Their findings are similar to what has been reported in the literature, that patients with Budd-Chiari syndrome can be successfully treated with TIPS as a bridge to liver transplantation, or even in lieu of surgical portosystemic shunting.
The authors have concluded that TIPS procedure is a safe, effective, and potentially long-lasting modality in the treatment of Budd-Chiari syndrome, especially for patients with high surgical risks, with paroxysmal nocturnal hemoglobinuria, and nontransplant candidates.
I have several questions for the authors. One, recent data from Europe suggest that TIPS patency may be improved with the use of PTFE-covered stent. Does your group have any experience with the use of covered stent for TIPS formation, and is there reason to believe this would improve results in Budd-Chiari syndrome?
Two, on average, how many and how often were revisions of the TIPS required? Presumably, the greater the number of revisions performed, the risk that this would interfere with liver transplantation if it is required.
The third question, you reported mean TIPS patency rate in patients with paroxysmal nocturnal hemoglobinuria was significantly shorter than TIPS mean patency rate in polycythemia as well as when it is done by open shunt procedures. Last February, Hillman and colleagues reported in the New England Journal of Medicine that a humanized chimeric monoclonal antibody against complement 5 prevented the hemolysis seen in patients with PNH. Is there reason to suspect this would help to prevent some of the thrombotic complications, such as the occlusions of TIPS?
Dr. C. Wright Pinson (Nashville, Tennessee): This is a very interesting experience. Clearly, TIPS provides good decompression in almost all patients. It is the patency that is the problem. And as such, I agree with the authors that TIPS must be used as a last resort in patients who won't tolerate surgical shunt or transplantation at the time.
For a comparison, it would be useful to not only know the number of TIPS that were performed in your institution in the same time period, but in that same 10 years how many patients at your institution went straight to surgical shunt and how many went straight to transplantation who had Budd-Chiari syndrome? Given the problems with patency, do you ever use anticoagulation to help with this?
You didn't mention it, but a strict follow-up protocol would be in order to maintain patency in these patients. I wonder if you can tell us what you would recommend for the follow-up on these TIPS procedures?
Finally, do you ever interrogate just the hepatic vein ostea for limited length stenosis and, if present, place a short stent just in that short segment of the hepatic vein rather than use a TIPS? I have read about a few case reports with this approach, and it seems like a nice alternative to the TIPS.
Dr. Max R. Langham, Jr. (Gainesville, Florida): Ienjoyed this paper a great deal and was interested in the age group of the patients that were described in this series. Budd-Chiari syndrome does occur in children, and in an institution like the Johns Hopkins Hospital, which has one of the premier bone marrow transplant units in the world, one suspects that they see a number of children of hepatic veno-occlusive disease that present with the Budd-Chiari physiology, and may see a number of cases in young adults from this and hemoglobinopathies.
My question for the authors concerns selection for inclusion in this series. Have they been consulted on other patients with the Budd-Chiari physiology who they have transplanted? What are their criteria for shunting or use of TIPS instead of them thinking about liver transplantation, particularly in young patients?
Dr. Ernesto P. Molmenti (Baltimore, Maryland): In reference to Dr. Zibari's question on covered stents, the current data show, as he very correctly mentioned, that covered stents are associated with a higher patency rate. I believe that in this respect they hold a very promising prospect for TIPS in the treatment of Budd-Chiari. We have a concern, however. And that concerns the fact that the PTFE covering of the stents in cases of malpositioning of the stent may lead to the occlusion of the adequate flow in certain cases.
In reference to the number of revisions, 2 of our patients had one revision, the remaining 4 had more than one revision. I think it is something to keep in mind. Although the patency of the TIPS according to the etiology of the Budd-Chiari syndrome may vary, I think that the length of the patency is also associated with the length of the follow-up in each individual case.
In reference to the complement 5 antibody study, complement 5 antibodies have been implicated in diminishing the amount of hemolysis in cases of paroxysmal nocturnal hemoglobineuria. Although at the present time we have no definite experience with Budd-Chiari and C5 antibodies, I think it holds very promising results.
In reference to Dr. Pinson's questions, over the same time period we performed 10 surgical shunts and approximately 8 liver transplants for Budd-Chiari. The 2 sets are not mutually exclusive, and some people who were shunted eventually progressed to receive liver transplants.
In reference to the other question, we believe that the treatment of Budd-Chiari syndrome as opposed to veno-occlusive disease syndrome is different; it varies; in each case, it should be treated accordingly.
In reference to Dr. Langham's question as to whether we performed other treatments, I think that the previous response addresses this, although we preferentially do shunts in Budd-Chiari patients, in cases of liver dysfunction we proceed with transplantation as well.
Footnotes
Reprints: Andrew Klein, MD, MBA, Cedars-Sinai Medical Center, 8635 W. Third St., Suite 590W, Los Angeles, CA 90048. E-mail: kleinas@cshs.org and emolmenti@yahoo.com.
REFERENCES
- 1.Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488–496. [DOI] [PubMed] [Google Scholar]
- 2.Valla DC. Hepatic vein thrombosis (Budd-Chiari syndrome). Semin Liver Dis. 2002;22:5–14. [DOI] [PubMed] [Google Scholar]
- 3.Orloff MJ, Daily PO, Orloff SL, et al. A 27-year experience with surgical treatment of Budd-Chiari syndrome. Ann Surg. 2000;232:340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein AS, Sitzmann JV, Coleman J, et al. Current management of the Budd-Chiari syndrome. Ann Surg. 1990;212:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein AS, Cameron JL. Diagnosis and management of the Budd-Chiari syndrome. Am J Surg. 1990;160:128–133. [DOI] [PubMed] [Google Scholar]
- 6.Panis Y, Belghiti J, Valla D, et al. Portosystemic shunt in Budd-Chiari syndrome: long-term survival and factors affecting shunt patency in 25 patients in Western countries. Surgery. 1994;115:276–281. [PubMed] [Google Scholar]
- 7.Halff G, Todo S, Tzakis AG, et al. Liver transplantation for the Budd-Chiari syndrome. Ann Surg. 1990;211:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan P, Rela M, Prachalias A, et al. Liver transplantation for Budd-Chiari syndrome. Transplantation. 2002;73:973–977. [DOI] [PubMed] [Google Scholar]
- 9.Pisani-Ceretti A, Intra M, Prestipino F, et al. Surgical and radiologic treatment of primary Budd-Chiari syndrome. World J Surg. 1998;22:48–53; discussion 53–54. [DOI] [PubMed]
- 10.Harris JW, Koscick R, Lazarus HM, et al. Leukemia arising out of paroxysmal nocturnal hemoglobinuria. Leuk Lymphoma. 1999;32:401–426. [DOI] [PubMed] [Google Scholar]
- 11.Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258. [DOI] [PubMed] [Google Scholar]
- 12.Slakey DP, Klein AS, Venbrux AC, et al. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233:522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosado B, Kamath PS. Transjugular intrahepatic portosystemic shunts: an update. Liver Transpl. 2003;9:207–217. [DOI] [PubMed] [Google Scholar]
- 14.Bilbao JI, Quiroga J, Herrero JI, et al. Transjugular intrahepatic portosystemic shunt (TIPS): current status and future possibilities. Cardiovasc Intervent Radiol. 2002;25:251–269. [DOI] [PubMed] [Google Scholar]
- 15.Cejna M, Peck-Radosavljevic M, Schoder M, et al. Repeat interventions for maintenance of transjugular intrahepatic portosystemic shunt function in patients with Budd-Chiari syndrome. J Vasc Interv Radiol. 2002;13:193–199. [DOI] [PubMed] [Google Scholar]
- 16.Blum U, Rossle M, Haag K, et al. Budd-Chiari syndrome: technical, hemodynamic, and clinical results of treatment with transjugular intrahepatic portosystemic shunt. Radiology. 1995;197:805–811. [DOI] [PubMed] [Google Scholar]
- 17.Ganger DR, Klapman JB, McDonald V, et al. Transjugular intrahepatic portosystemic shunt (TIPS) for Budd-Chiari syndrome or portal vein thrombosis: review of indications and problems. Am J Gastroenterol. 1999;94:603–608. [DOI] [PubMed] [Google Scholar]
- 18.Gasparini D, Del Forno M, Sponza M, et al. Transjugular intrahepatic portosystemic shunt by direct transcaval approach in patients with acute and hyperacute Budd-Chiari syndrome. Eur J Gastroenterol Hepatol. 2002;14:567–571. [DOI] [PubMed] [Google Scholar]
- 19.Ochs A, Sellinger M, Haag K, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPS) in the treatment of Budd-Chiari syndrome. J Hepatol. 1993;18:217–225. [DOI] [PubMed] [Google Scholar]
- 20.Perello A, Garcia-Pagan JC, Gilabert R, et al. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medical therapy. Hepatology. 2002;35:132–139. [DOI] [PubMed] [Google Scholar]
- 21.Ryu RK, Durham JD, Krysl J, et al. Role of TIPS as a bridge to hepatic transplantation in Budd-Chiari syndrome. J Vasc Interv Radiol. 1999;10:799–805. [DOI] [PubMed] [Google Scholar]
- 22.Burroughs AK, Patch D. Transjugular intrahepatic portosystemic shunt. Semin Liver Dis. 1999;19:457–473. [DOI] [PubMed] [Google Scholar]