Abstract
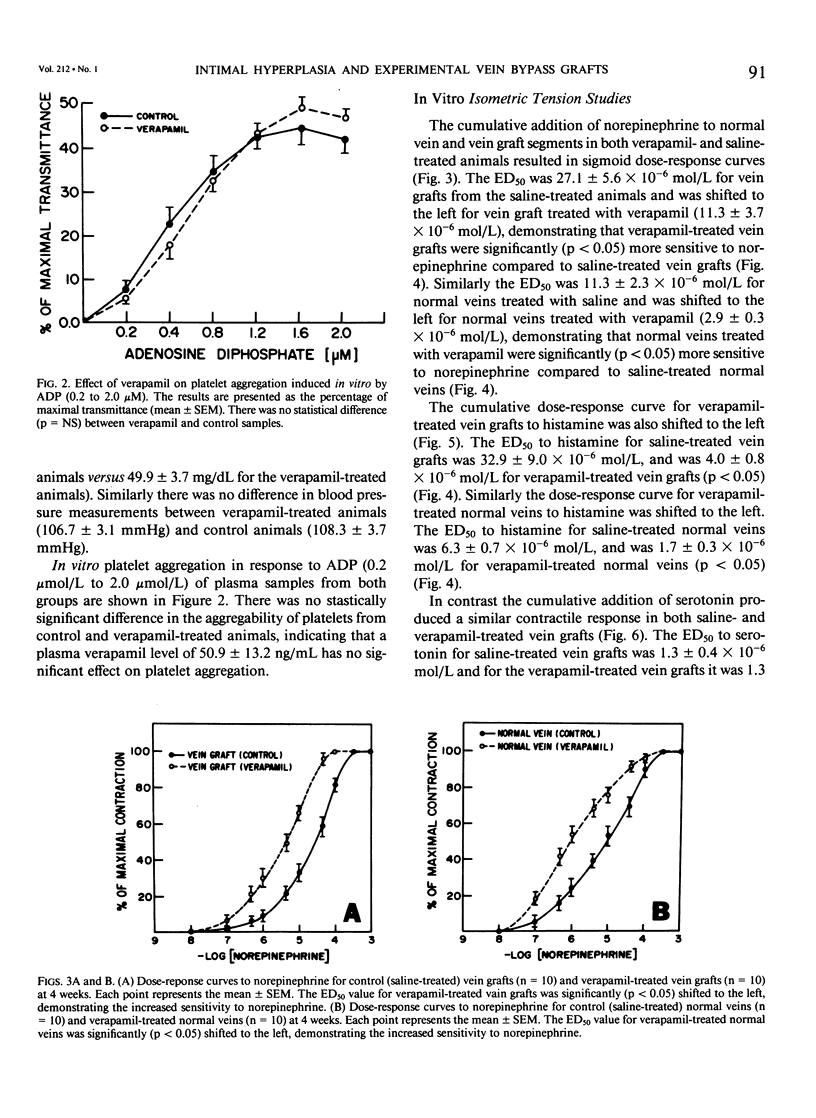
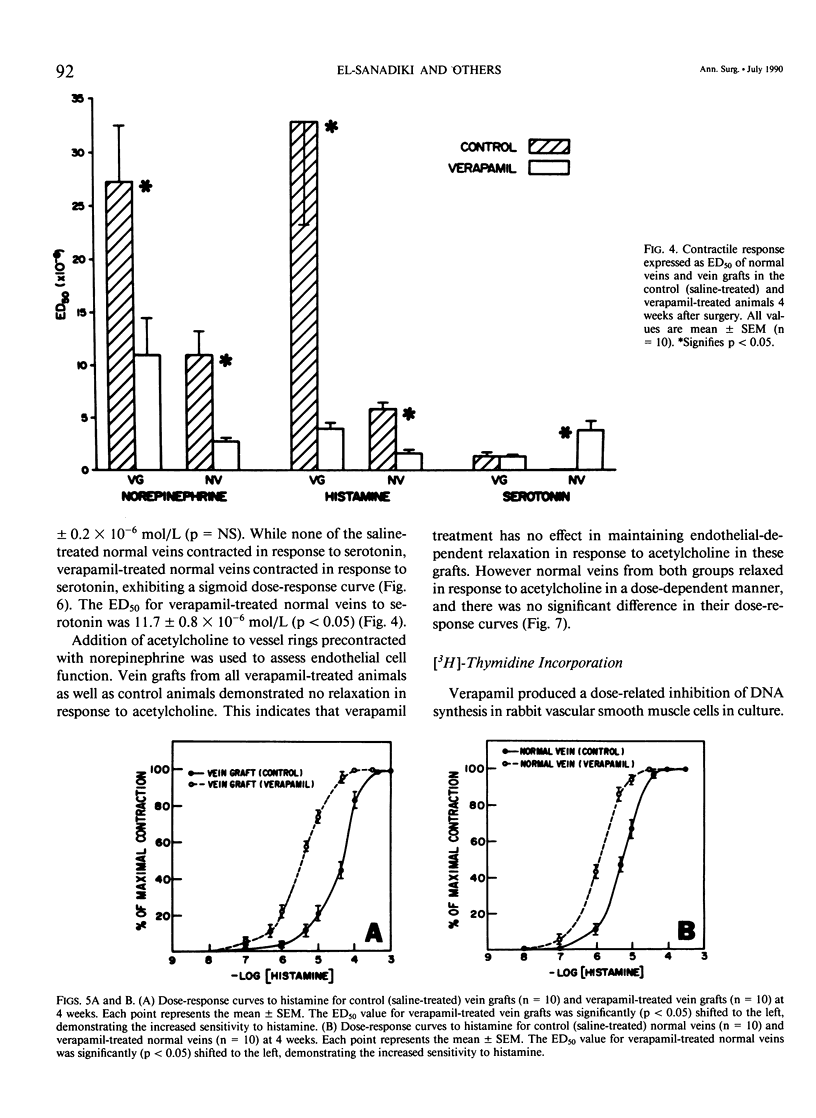
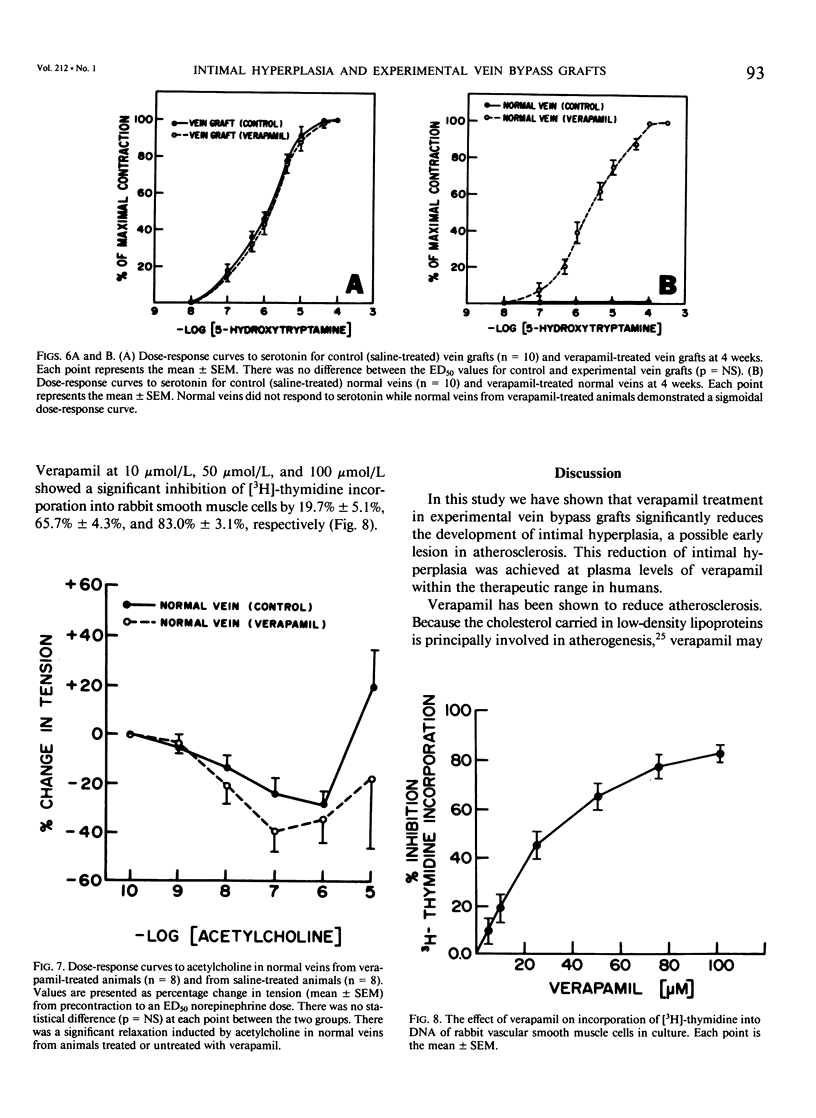
Recent studies have shown that calcium antagonists exert an antiatherogenic effect in animals fed cholesterol. Vein graft intimal hyperplasia is believed to be an early event in atherosclerotic lesion formation, which is a significant cause of graft failure. Altered vasoreactivity has also been postulated in the etiology of vein graft failure. Therefore this study examined the effect of verapamil treatment on the development of intimal hyperplasia and the vasoreactivity of experimental vein bypass grafts. The right external jugular vein was grafted into the right carotid artery of 30 male New Zealand white rabbits fed normal rabbit chow. The left external jugular vein was used as the control vein. Fifteen animals received verapamil (1.25 mg/day for 28 days) via the femoral vein by means of an osmotic pump. In 15 control animals the pump contained saline. Plasma verapamil concentration was 50.9 +/- 13.2 ng/mL (x +/- SEM), a dose that showed no effect on either blood pressure, total serum cholesterol, or in vitro platelet aggregation to ADP. Fourteen of fifteen grafts were patent in each group, for a patency rate of 93%. Histologic examination using computer morphometry showed significant reduction of intimal hyperplasia at the proximal, middle, and distal graft segments (p less than 0.05). In addition in vitro isometric tension studies of the vein grafts and control veins showed that verapamil causes enhanced reactivity of both vein grafts and control veins in response to norepinephrine and histamine (p less than 0.05). Reactivity of vein grafts to serotonin was unaltered. While none of the normal veins in the control group responded to serotonin, normal veins treated with verapamil contracted readily in response to serotonin. Endothelial-dependent relaxation to acetylcholine was absent in both control and verapamil-treated vein grafts, while normal veins from both groups responded to the same extent to acetylcholine. Because we could not demonstrate any difference in platelet or endothelium function between untreated and verapamil-treated animals, we examined the direct effect of verapamil on smooth muscle. Verapamil significantly inhibited [3H]-thymidine incorporation into DNA in vascular smooth muscle cells in culture in a dose-dependent manner. Verapamil treatment significantly reduces intimal hyperplasia in experimental vein grafts and inhibits smooth muscle cell proliferation in culture. Furthermore the enhanced reactivity to norepinephrine and histamine in the verapamil-treated vessels has no detrimental effect on the patency rate at 4 weeks. Thus by inhibiting intimal hyperplasia, calcium antagonists may improve the long-term patency of vein bypass grafts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnathan E. S., Addonizio V. P., Shattil S. J. Interaction of verapamil with human platelet alpha-adrenergic receptors. Am J Physiol. 1982 Jan;242(1):H19–H23. doi: 10.1152/ajpheart.1982.242.1.H19. [DOI] [PubMed] [Google Scholar]
- Blumlein S. L., Sievers R., Kidd P., Parmley W. W. Mechanism of protection from atherosclerosis by verapamil in the cholesterol-fed rabbit. Am J Cardiol. 1984 Oct 1;54(7):884–889. doi: 10.1016/s0002-9149(84)80226-x. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Bush H. L., Jr, Jakubowski J. A., Curl G. R., Deykin D., Nabseth D. C. The natural history of endothelial structure and function in arterialized vein grafts. J Vasc Surg. 1986 Feb;3(2):204–215. doi: 10.1067/mva.1986.avs0030204. [DOI] [PubMed] [Google Scholar]
- CHIAMORI N., HENRY R. J. Study of the ferric chloride method for determination of total cholesterol and cholesterol esters. Am J Clin Pathol. 1959 Apr;31(4):305–309. doi: 10.1093/ajcp/31.4.305. [DOI] [PubMed] [Google Scholar]
- Cole C. W., Hagen P. O., Lucas J. F., Mikat E. M., O'Malley M. K., Radic Z. S., Makhoul R. G., McCann R. L. Association of polymorphonuclear leukocytes with sites of aortic catheter-induced injury in rabbits. Atherosclerosis. 1987 Oct;67(2-3):229–236. doi: 10.1016/0021-9150(87)90283-8. [DOI] [PubMed] [Google Scholar]
- Cole C. W., Makhoul R. G., McCann R. L., O'Malley M. K., Hagen P. O. A neutrophil derived factor(s) stimulates [3H]thymidine incorporation by vascular smooth muscle cells in vitro. Clin Invest Med. 1988 Feb;11(1):62–67. [PubMed] [Google Scholar]
- Conti C. R., Pepine C. J., Feldman R. L., Hill J. A. Calcium antagonists. Cardiology. 1985;72(5-6):297–321. doi: 10.1159/000173886. [DOI] [PubMed] [Google Scholar]
- Etingin O. R., Hajjar D. P. Nifedipine increases cholesteryl ester hydrolytic activity in lipid-laden rabbit arterial smooth muscle cells. A possible mechanism for its antiatherogenic effect. J Clin Invest. 1985 May;75(5):1554–1558. doi: 10.1172/JCI111860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg R., Davis K., Bristow M. R., McKennett K., Kodsi S. R., Billingham M. E., Schroeder J. S. Calcium antagonists suppress atherogenesis in aorta but not in the intramural coronary arteries of cholesterol-fed rabbits. Lab Invest. 1983 Aug;49(2):154–158. [PubMed] [Google Scholar]
- Glossmann H., Hornung R. Calcium- and potassium-channel blockers interact with alpha-adrenoceptors. Mol Cell Endocrinol. 1980 Sep;19(3):243–251. doi: 10.1016/0303-7207(80)90054-4. [DOI] [PubMed] [Google Scholar]
- Groves H. M., Kinlough-Rathbone R. L., Mustard J. F. Development of nonthrombogenicity of injured rabbit aortas despite inhibition of platelet adherence. Arteriosclerosis. 1986 Mar-Apr;6(2):189–195. doi: 10.1161/01.atv.6.2.189. [DOI] [PubMed] [Google Scholar]
- Gundry S. R., Jones M., Ishihara T., Ferrans V. J. Intraoperative trauma to human saphenous veins: scanning electron microscopic comparison of preparation techniques. Ann Thorac Surg. 1980 Jul;30(1):40–47. doi: 10.1016/s0003-4975(10)61200-3. [DOI] [PubMed] [Google Scholar]
- Habib J. B., Bossaller C., Wells S., Williams C., Morrisett J. D., Henry P. D. Preservation of endothelium-dependent vascular relaxation in cholesterol-fed rabbit by treatment with the calcium blocker PN 200110. Circ Res. 1986 Feb;58(2):305–309. doi: 10.1161/01.res.58.2.305. [DOI] [PubMed] [Google Scholar]
- Han P., Boatwright C., Ardlie N. G. Verapamil and collagen-induced platelet reactions--evidence for a role for intracellular calcium in platelet activation. Thromb Haemost. 1983 Aug 30;50(2):537–540. [PubMed] [Google Scholar]
- Handley D. A., Van Valen R. G., Melden M. K., Saunders R. N. Suppression of rat carotid lesion development by the calcium channel blocker PN 200-110. Am J Pathol. 1986 Jul;124(1):88–93. [PMC free article] [PubMed] [Google Scholar]
- Harapat S. R., Kates R. E. Rapid high-pressure liquid chromatographic analysis of verapamil in blood and plasma. J Chromatogr. 1979 Mar 11;170(2):385–390. doi: 10.1016/s0021-9673(00)95464-5. [DOI] [PubMed] [Google Scholar]
- Henry P. D., Bentley K. I. Suppression of atherogenesis in cholesterol-fed rabbit treated with nifedipine. J Clin Invest. 1981 Nov;68(5):1366–1369. doi: 10.1172/JCI110384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Kikuchi M., Toyama K., Watanabe K., Ando Y. Inhibition of human platelet functions by verapamil. Thromb Haemost. 1981 Apr 30;45(2):158–161. [PubMed] [Google Scholar]
- Jackson C. L., Bush R. C., Bowyer D. E. Inhibitory effect of calcium antagonists on balloon catheter-induced arterial smooth muscle cell proliferation and lesion size. Atherosclerosis. 1988 Feb;69(2-3):115–122. doi: 10.1016/0021-9150(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Karliner J. S., Motulsky H. J., Dunlap J., Brown J. H., Insel P. A. Verapamil competitively inhibits alpha 1-adrenergic and muscarinic but not beta-adrenergic receptors in rat myocardium. J Cardiovasc Pharmacol. 1982 May-Jun;4(3):515–520. doi: 10.1097/00005344-198205000-00025. [DOI] [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Murray J. J., Fridovich I., Makhoul R. G., Hagen P. O. Stabilization and partial characterization of endothelium-derived relaxing factor from cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):689–696. doi: 10.1016/s0006-291x(86)80227-3. [DOI] [PubMed] [Google Scholar]
- Nakao J., Ito H., Ooyama T., Chang W. C., Murota S. Calcium dependency of aortic smooth muscle cell migration induced by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. Effects of A23187, nicardipine and trifluoperazine. Atherosclerosis. 1983 Mar;46(3):309–319. doi: 10.1016/0021-9150(83)90180-6. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Von Euler A. M., Jonzon B., Thyberg J. The calcium antagonist nifedipine inhibits arterial smooth muscle cell proliferation. Atherosclerosis. 1985 Dec;58(1-3):109–122. doi: 10.1016/0021-9150(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Hirosumi J., Sekiguchi C., Mutoh S., Yamaguchi I., Aoki H. Antiatherogenic activity of FR34235 (Nilvadipine), a new potent calcium antagonist. Effect on cuff-induced intimal thickening of rabbit carotid artery. Atherosclerosis. 1987 Apr;64(2-3):255–261. doi: 10.1016/0021-9150(87)90253-x. [DOI] [PubMed] [Google Scholar]
- Okumura K., Yasue H., Horio Y., Takaoka K., Matsuyama K., Kugiyama K., Fujii H., Morikami Y. Multivessel coronary spasm in patients with variant angina: a study with intracoronary injection of acetylcholine. Circulation. 1988 Mar;77(3):535–542. doi: 10.1161/01.cir.77.3.535. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Srivastava K. C. Effects of dipyridamole, nifedipine, verapamil, hydralazine and propranolol on the formation of prostacyclin and thromboxane in a coupled system of platelets and aorta. Prostaglandins Leukot Med. 1986 Jul;23(1):31–36. doi: 10.1016/0262-1746(86)90074-0. [DOI] [PubMed] [Google Scholar]
- Stein O., Halperin G., Stein Y. Long-term effects of verapamil on aortic smooth muscle cells cultured in the presence of hypercholesterolemic serum. Arteriosclerosis. 1987 Nov-Dec;7(6):585–592. doi: 10.1161/01.atv.7.6.585. [DOI] [PubMed] [Google Scholar]
- Stein O., Leitersdorf E., Stein Y. Verapamil enhances receptor-mediated endocytosis of low density lipoproteins by aortic cells in culture. Arteriosclerosis. 1985 Jan-Feb;5(1):35–44. doi: 10.1161/01.atv.5.1.35. [DOI] [PubMed] [Google Scholar]
- Steiner R. D., Pratt A., Busse W. W. Cytochalasin B facilitates the inhibition of human polymorphonuclear leukocyte generation of superoxide by verapamil. J Lab Clin Med. 1984 Jun;103(6):949–958. [PubMed] [Google Scholar]
- Stender S., Ravn H., Haugegaard M., Kjeldsen K. Effect of verapamil on accumulation of free and esterified cholesterol in the thoracic aorta of cholesterol-fed rabbits. Atherosclerosis. 1986 Jul;61(1):15–23. doi: 10.1016/0021-9150(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Sugano M., Nakashima Y., Matsushima T., Takahara K., Takasugi M., Kuroiwa A., Koide O. Suppression of atherosclerosis in cholesterol-fed rabbits by diltiazem injection. Arteriosclerosis. 1986 Mar-Apr;6(2):237–241. doi: 10.1161/01.atv.6.2.237. [DOI] [PubMed] [Google Scholar]
- Verstraete M., Brown B. G., Chesebro J. H., Ekeström S., Harker L. A., Henderson A. H., Jewitt D. E., Oliver M. F., Sleight P. Evaluation of antiplatelet agents in the prevention of aorto-coronary bypass occlusion. Eur Heart J. 1986 Jan;7(1):4–13. doi: 10.1093/oxfordjournals.eurheartj.a061955. [DOI] [PubMed] [Google Scholar]
- Weinstein D. B., Heider J. G. Antiatherogenic properties of calcium antagonists. Am J Cardiol. 1987 Jan 30;59(3):163B–172B. doi: 10.1016/0002-9149(87)90097-x. [DOI] [PubMed] [Google Scholar]



