Abstract
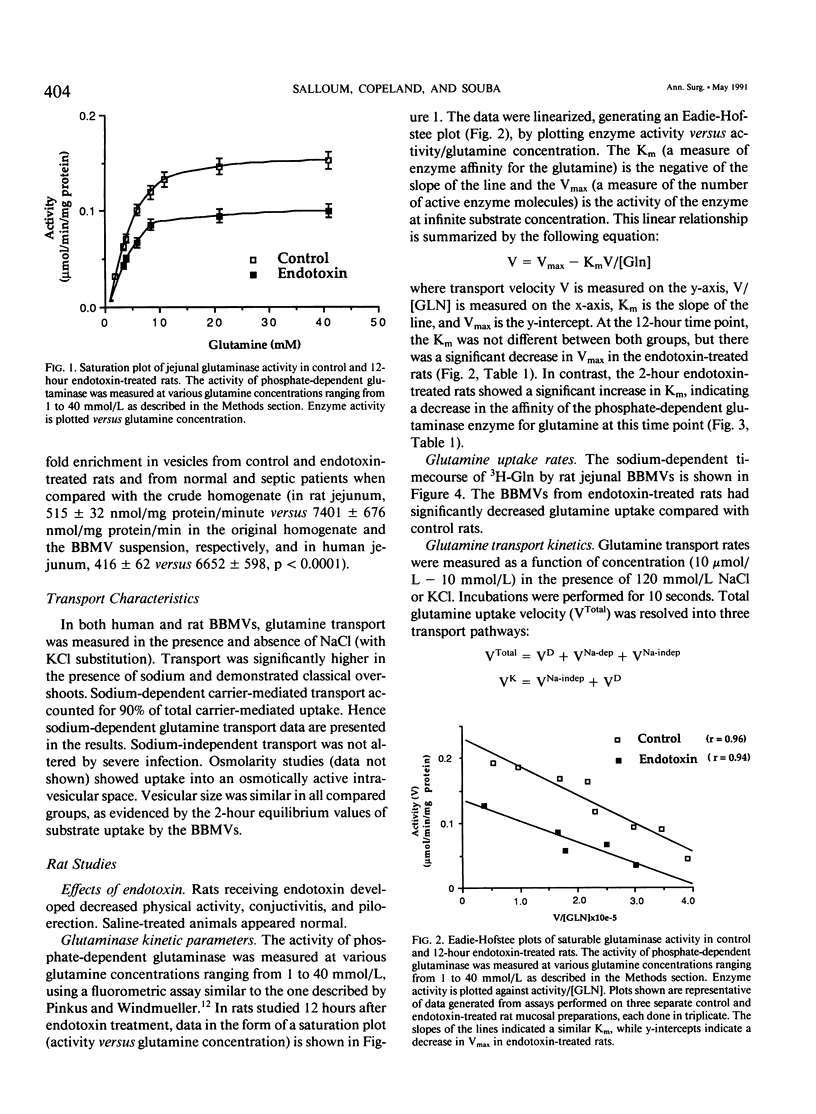
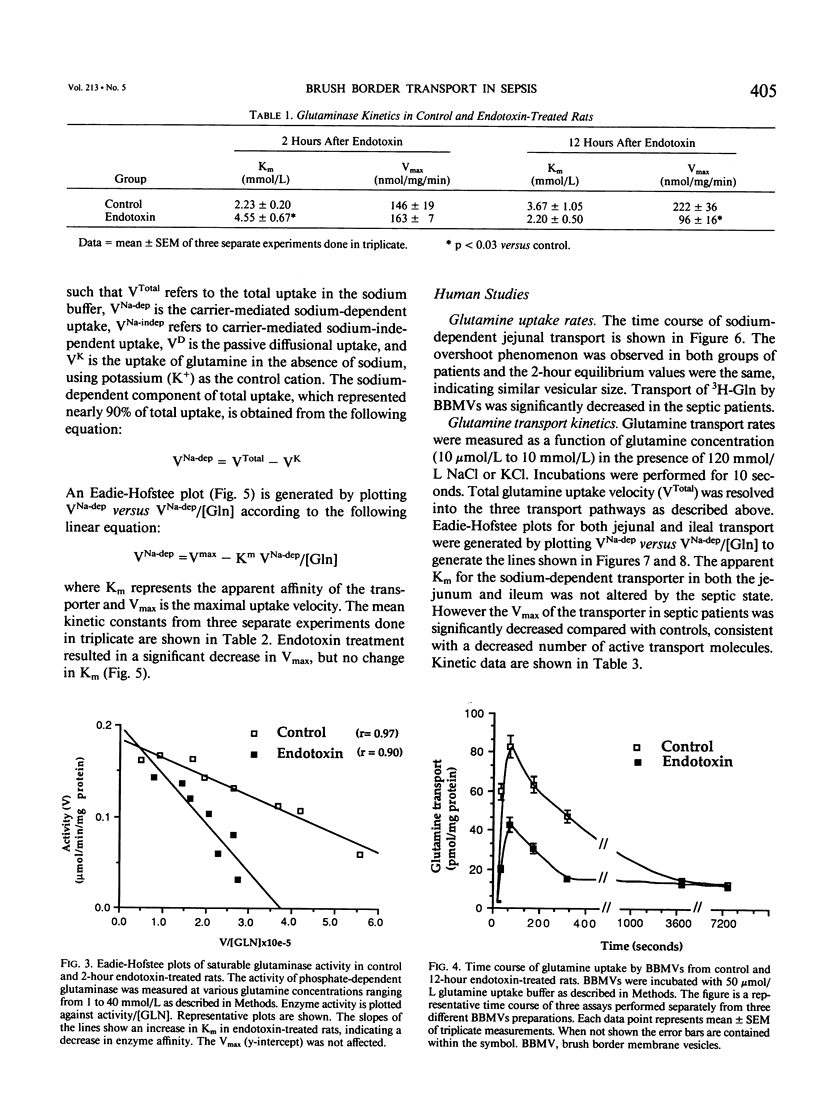
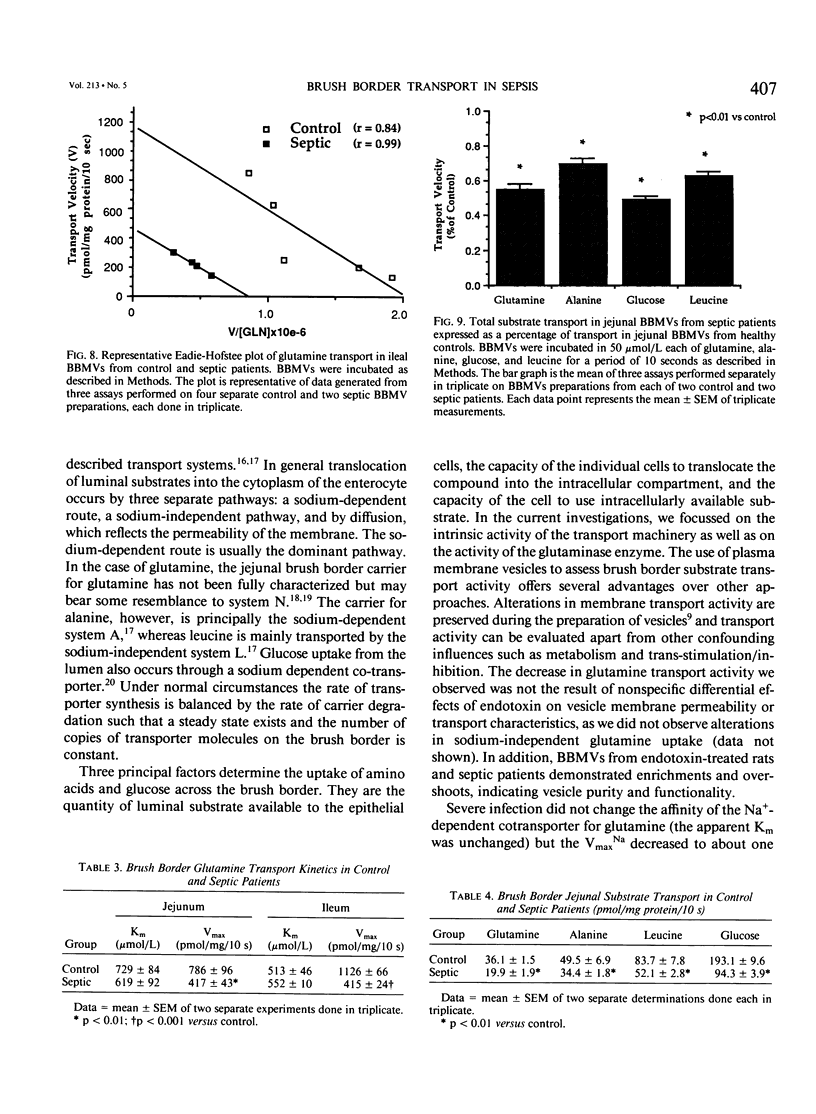
The effects of severe infection on luminal transport of amino acids and glucose by the small intestine were investigated. Studies were done in endotoxin-treated rats and in septic patients who underwent resection of otherwise normal small bowel. In rats the kinetics of the brush border glutamine transporter and the glutaminase enzyme were examined. In patients the effects of severe infection on the transport of glutamine, alanine, leucine, and glucose were studied. Transport was measured using small intestinal brush border membrane vesicles that were prepared by Mg++ aggregation/differential centrifugation. Uptake of radiolabeled substrate was measured using a rapid mixing/filtration technique. Vesicles demonstrated 15-fold enrichments of enzyme markers, classic overshoots, transport into an osmotically active space, and similar 2-hour equilibrium values. The sodium-dependent pathway accounted for nearly 90% of total carrier-mediated transport. Kinetic studies on rat jejunal glutaminase indicated a decrease in activity as early as 2 hours after endotoxin secondary to a decrease in enzyme affinity for glutamine (Km = 2.23 +/- 0.20 mmol/L [millimolar] in controls versus 4.55 +/- 0.67 in endotoxin, p less than 0.03), rather than a change in Vmax. By 12 hours the decrease in glutaminase activity was due to a decrease in Vmax (222 +/- 36 nmol/mg protein/min in controls versus 96 +/- 16 in endotoxin, p less than 0.03) rather than a significant change in Km. Transport data indicated a decrease in sodium-dependent jejunal glutamine uptake 12 hours after endotoxin secondary to a 35% reduction in maximal transport velocity (Vmax = 325 +/- 12 pmol/mg protein/10 sec in controls versus 214 +/- 8 in endotoxin, p less than 0.0001) with no change in Km (carrier affinity). Sodium-dependent glutamine transport was also decreased in septic patients, both in the jejunum (Vmax for control jejunum = 786 +/- 96 pmol/mg protein/10 sec versus 417 +/- 43 for septic jejunum, p less than 0.01) and in the ileum (Vmax of control ileum = 1126 +/- 66 pmol/mg protein/10 sec versus 415 +/- 24 in septic ileum, p less than 0.001) The rate of jejunal transport of alanine, leucine, and glucose was also decreased in septic patients by 30% to 50% (p less than 0.01). These data suggest that there is a generalized down-regulation of sodium-dependent carrier-mediated substrate transport across the brush border during severe infection, which probably occurs secondary to a decrease in transporter synthesis or an increase in the rate of carrier degradation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström J., Fürst P., Norée L. O., Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974 Jun;36(6):693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Berg R., Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987 Feb;122(2):185–190. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Karasov W. H. Adaptive regulation of intestinal nutrient transporters. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2242–2245. doi: 10.1073/pnas.84.8.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J. M. Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu Rev Physiol. 1989;51:125–141. doi: 10.1146/annurev.ph.51.030189.001013. [DOI] [PubMed] [Google Scholar]
- Fink M. P., Heard S. O. Laboratory models of sepsis and septic shock. J Surg Res. 1990 Aug;49(2):186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- Fox A. D., Kripke S. A., De Paula J., Berman J. M., Settle R. G., Rombeau J. L. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr. 1988 Jul-Aug;12(4):325–331. doi: 10.1177/0148607188012004325. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Groseclose R. The mechanism of Na+-dependent D-glucose transport. J Biol Chem. 1980 May 25;255(10):4453–4462. [PubMed] [Google Scholar]
- Klimberg V. S., Salloum R. M., Kasper M., Plumley D. A., Dolson D. J., Hautamaki R. D., Mendenhall W. R., Bova F. C., Bland K. I., Copeland E. M., 3rd Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg. 1990 Aug;125(8):1040–1045. doi: 10.1001/archsurg.1990.01410200104017. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Souba W. W., Dolson D. J., Salloum R. M., Hautamaki R. D., Plumley D. A., Mendenhall W. M., Bova F. J., Khan S. R., Hackett R. L. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990 Jul 1;66(1):62–68. doi: 10.1002/1097-0142(19900701)66:1<62::aid-cncr2820660113>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Moore F. A., Moore E. E., Jones T. N., McCroskey B. L., Peterson V. M. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989 Jul;29(7):916–923. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- Parry-Billings M., Leighton B., Dimitriadis G., de Vasconcelos P. R., Newsholme E. A. Skeletal muscle glutamine metabolism during sepsis in the rat. Int J Biochem. 1989;21(4):419–423. doi: 10.1016/0020-711x(89)90366-2. [DOI] [PubMed] [Google Scholar]
- Peterson V. M., Moore E. E., Jones T. N., Rundus C., Emmett M., Moore F. A., McCroskey B. L., Haddix T., Parsons P. E. Total enteral nutrition versus total parenteral nutrition after major torso injury: attenuation of hepatic protein reprioritization. Surgery. 1988 Aug;104(2):199–207. [PubMed] [Google Scholar]
- Pinkus L. M., Windmueller H. G. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. 1977 Aug;182(2):506–517. doi: 10.1016/0003-9861(77)90531-8. [DOI] [PubMed] [Google Scholar]
- Said H. M., Van Voorhis K., Ghishan F. K., Abumurad N., Nylander W., Redha R. Transport characteristics of glutamine in human intestinal brush-border membrane vesicles. Am J Physiol. 1989 Jan;256(1 Pt 1):G240–G245. doi: 10.1152/ajpgi.1989.256.1.G240. [DOI] [PubMed] [Google Scholar]
- Salloum R. M., Souba W. W., Fernandez A., Stevens B. R. Dietary modulation of small intestinal glutamine transport in intestinal brush border membrane vesicles of rats. J Surg Res. 1990 Jun;48(6):635–638. doi: 10.1016/0022-4804(90)90244-v. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Kilberg M. S., Oxender D. L. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Herskowitz K., Klimberg V. S., Salloum R. M., Plumley D. A., Flynn T. C., Copeland E. M., 3rd The effects of sepsis and endotoxemia on gut glutamine metabolism. Ann Surg. 1990 May;211(5):543–551. doi: 10.1097/00000658-199005000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souba W. W., Klimberg V. S., Plumley D. A., Salloum R. M., Flynn T. C., Bland K. I., Copeland E. M., 3rd The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res. 1990 Apr;48(4):383–391. doi: 10.1016/0022-4804(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Kaunitz J. D., Wright E. M. Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annu Rev Physiol. 1984;46:417–433. doi: 10.1146/annurev.ph.46.030184.002221. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Ross H. J., Wright E. M. Multiple transport pathways for neutral amino acids in rabbit jejunal brush border vesicles. J Membr Biol. 1982;66(3):213–225. doi: 10.1007/BF01868496. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Smith R. J., O'Dwyer S. T., Jacobs D. O., Ziegler T. R., Wang X. D. The gut: a central organ after surgical stress. Surgery. 1988 Nov;104(5):917–923. [PubMed] [Google Scholar]


