Abstract
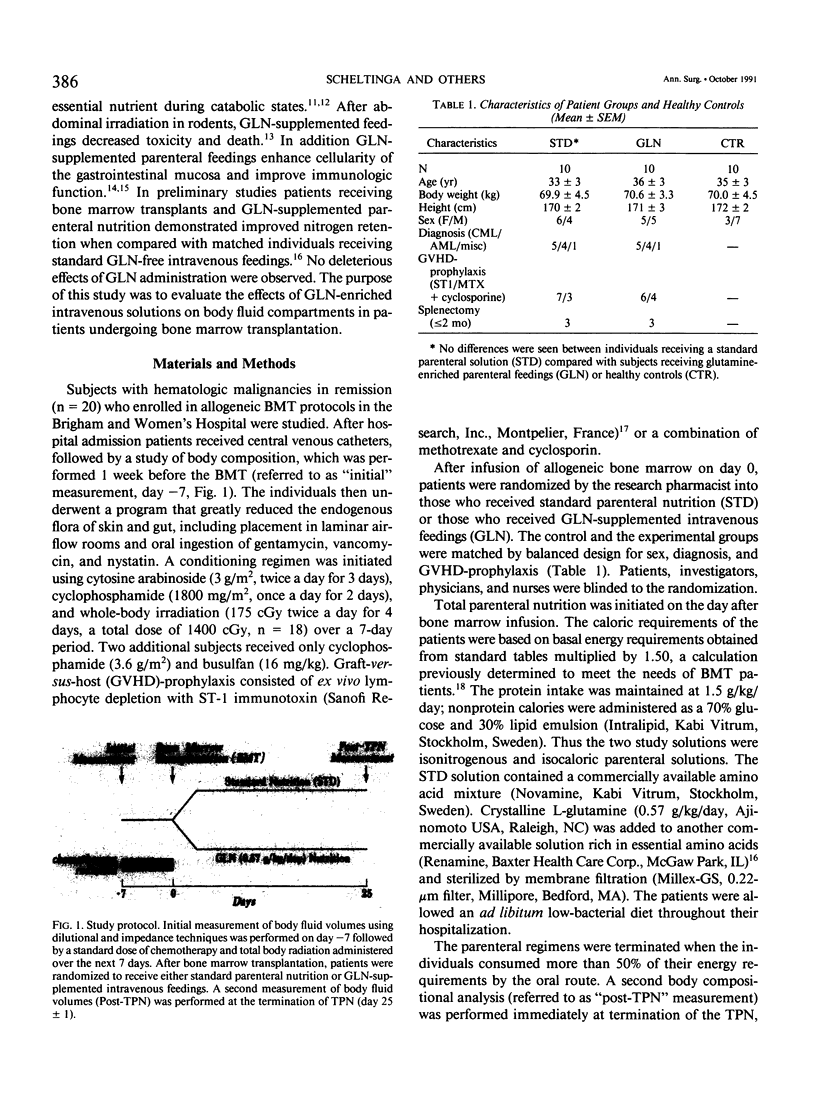
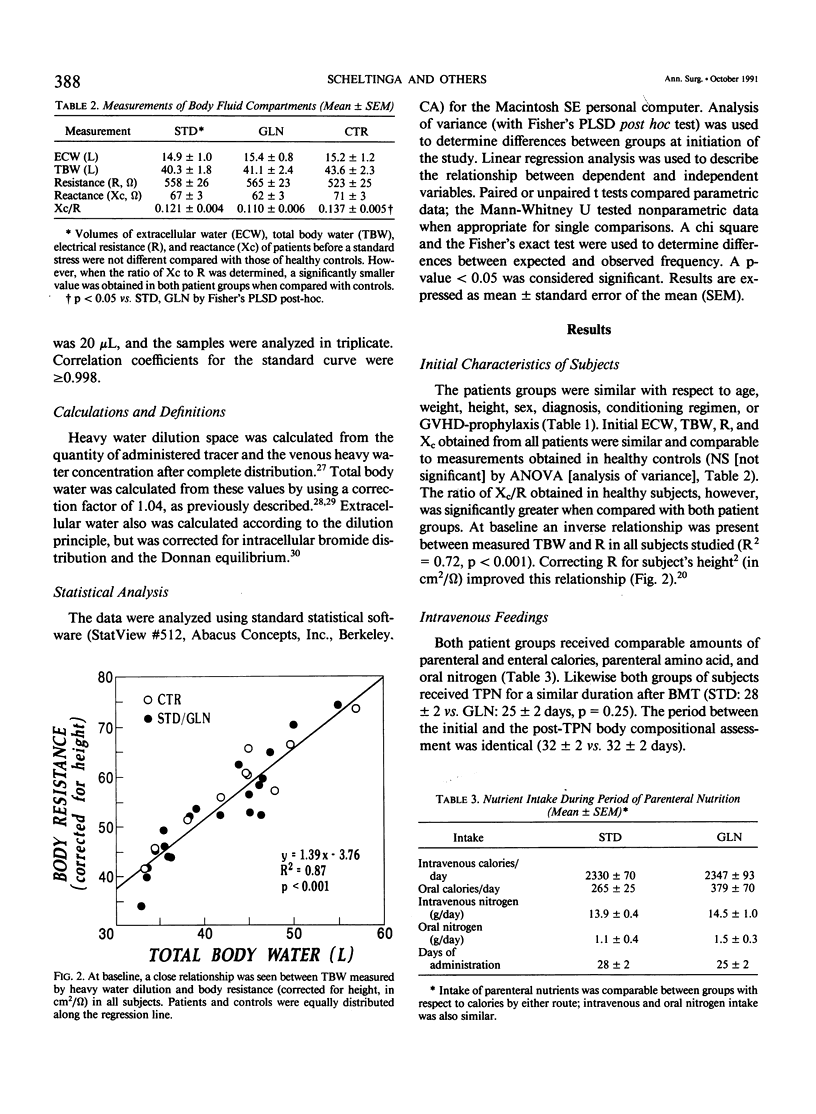
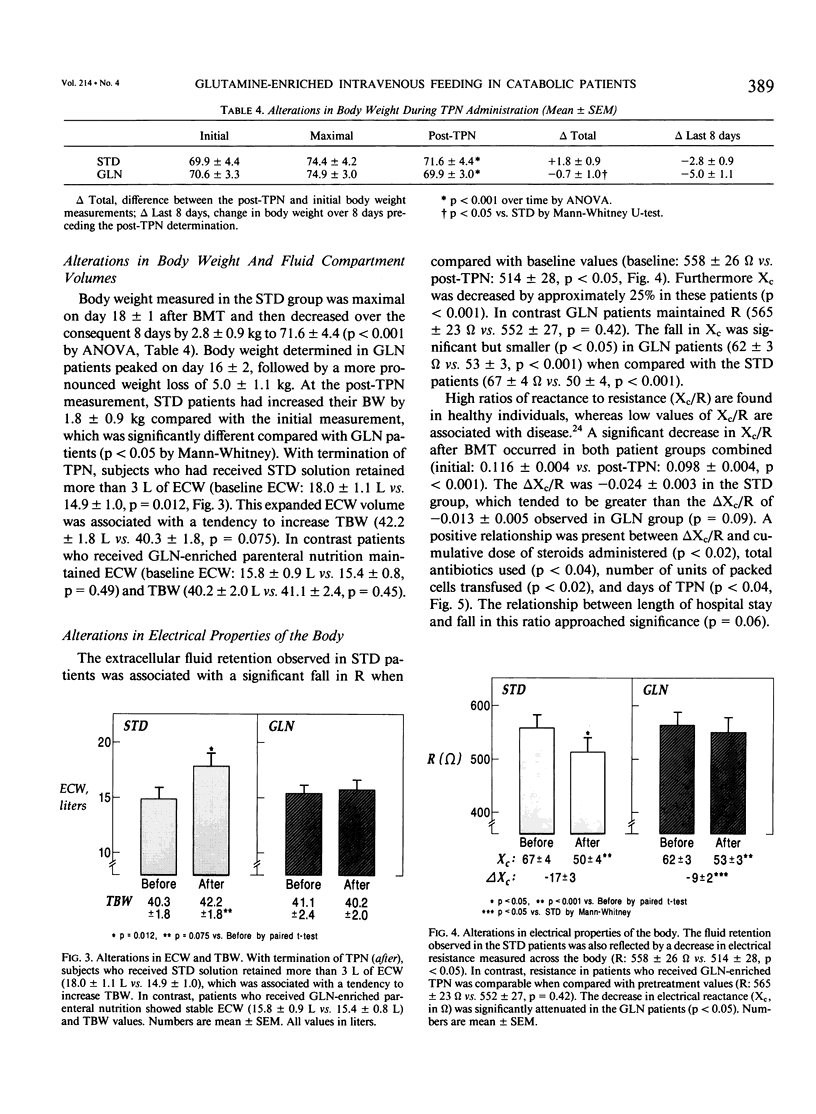
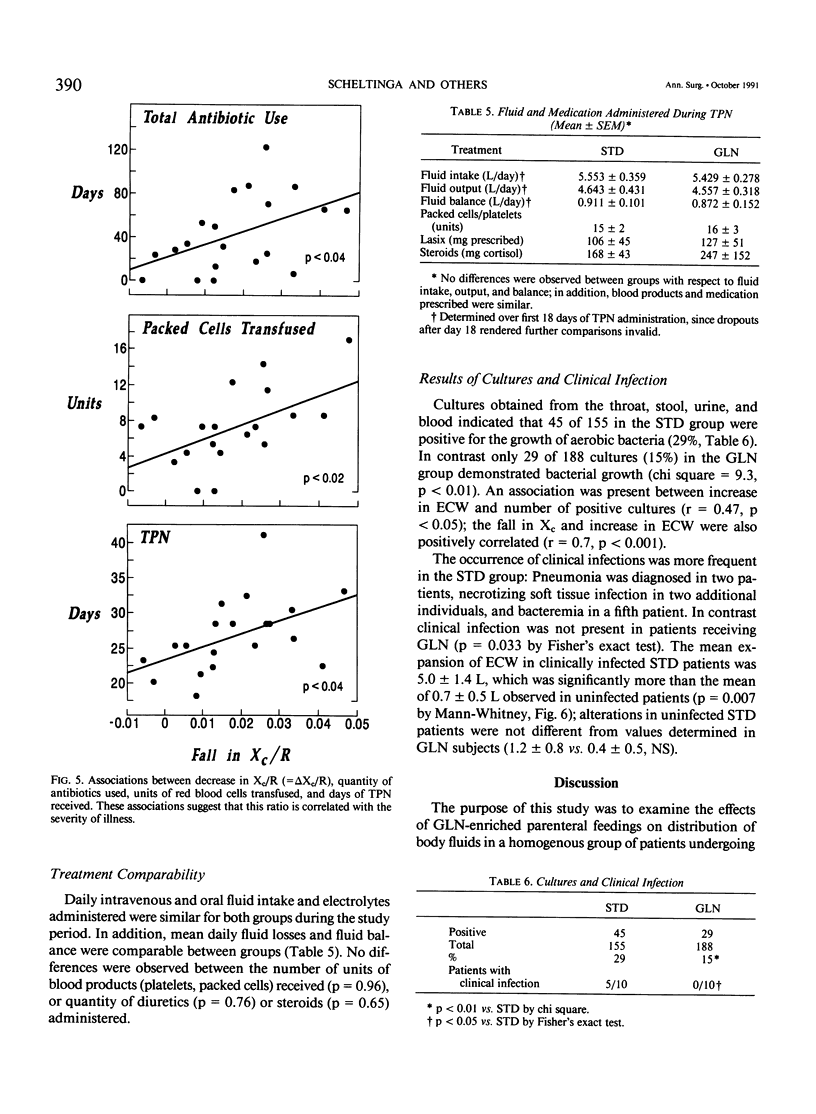
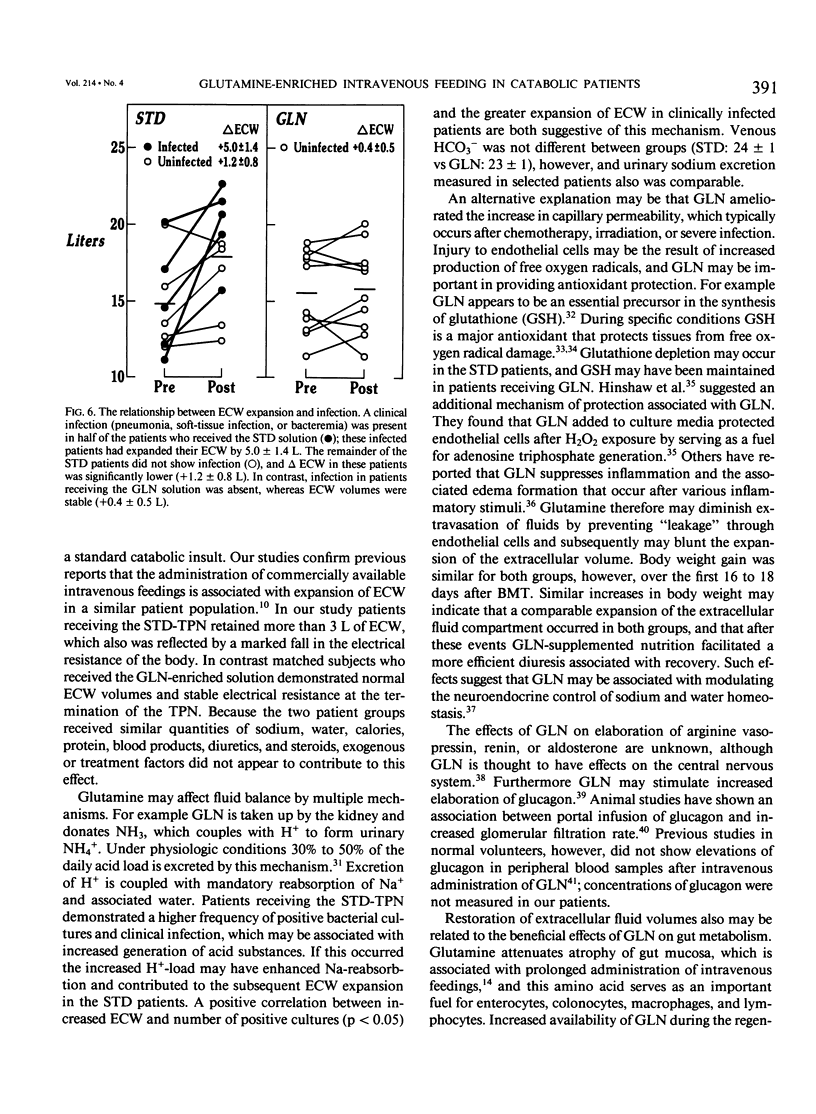
A double-blind, randomized controlled trial was performed to determine the effect of glutamine (GLN)-enriched intravenous feedings on the volume and distribution of body fluids in catabolic patients. Subjects with hematologic malignancies in remission underwent a standard treatment of high-dose chemotherapy and total body irradiation before bone marrow transplantation. After completion of this regimen, they were randomized to receive either standard parenteral nutrition (STD, n = 10) or an isocaloric, isonitrogenous nutrient solution enriched with crystalline L-glutamine (0.57 g/kg/day, GLN, n = 10). Extracellular water (ECW) and total body water (TBW), determined by bromide and heavy water dilution techniques, were measured before the conditioning treatment and after termination of the intravenous feedings that were administered for 27 +/- 1 days. In addition electrical resistance (R, in ohms, omega) and reactance (Xc, omega) of the body to a weak alternating current were measured at these time points. Both study groups were comparable for age, weight, height, sex, and diagnosis. Initial TBW was highly related to electrical resistance (r = -0.93, p less than 0.001). After conditioning therapy, bone marrow infusion, and intravenous feedings, a 20% expansion in ECW was observed in the STD group (ECW: 18.0 +/- 1.1 L vs. 14.9 +/- 1.0, p = 0.012), and this fluid retention was associated with a marked decrease in electrical resistance (R: 514 +/- 28 omega vs. 558 +/- 26, p less than 0.05). In contrast the extracellular fluid compartment in patients receiving GLN-supplementation did not change (ECW: 15.8 +/- 0.9 L vs. 15.4 +/- 0.8, p = 0.49), and the body's resistance was maintained (R: 552 +/- 27 omega vs. 565 +/- 23, p = 0.42). Expansion of ECW could not be related to differences in fluid or sodium intake, or to the use of diuretics or steroids. Patients receiving the STD solution, however, exhibited a greater number of positive microbial cultures (p less than 0.01) and a higher rate of clinical infection compared with the GLN patients (5/10 vs. 0/10, p less than 0.05); the fluid expansion in infected STD patients was greater compared with uninfected individuals (delta ECW: + 5.0 +/- 1.4 vs. 0.7 +/- 0.5, p = 0.007). In this model of catabolic stress, fluid retention and expansion of the extracellular fluid compartment commonly observed after standard total parenteral nutrition can be attenuated by administering glutamine-supplemented intravenous feedings, possibly by protecting the host from microbial invasion and associated infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antin J. H., Bierer B. E., Smith B. R., Guinan E. C., Provost M. M., Ferrara J., Macklis R. M., Tarbell N. J., Blythman H., Bouloux C. Depletion of bone marrow T-lymphocytes with an anti-CD5 monoclonal immunotoxin (ST-1 immunotoxin): effective prophylaxis for graft-versus-host disease. Prog Clin Biol Res. 1990;333:207–215. [PubMed] [Google Scholar]
- Bell E. F., Ziegler E. E., Forbes G. B. Corrected bromide space. Pediatr Res. 1984 Apr;18(4):392–393. doi: 10.1203/00006450-198404000-00021. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Alverdy J. C., Aoys E., Moss G. S. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg. 1989 Dec;124(12):1396–1399. doi: 10.1001/archsurg.1989.01410120042009. [DOI] [PubMed] [Google Scholar]
- Böck J. C., Barker B. C., Clinton A. G., Wilson M. B., Lewis F. R. Post-traumatic changes in, and effect of colloid osmotic pressure on the distribution of body water. Ann Surg. 1989 Sep;210(3):395–405. doi: 10.1097/00000658-198909000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. L., Abson K. G., Aker S. N., Lenssen P., Cunningham B. A., Buergel N. S., Thomas E. D. Body composition changes in marrow transplant recipients receiving total parenteral nutrition. Cancer. 1987 Apr 15;59(8):1515–1519. doi: 10.1002/1097-0142(19870415)59:8<1515::aid-cncr2820590821>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cocchi R. Antidepressive properties of I-glutamine. Preliminary report. Acta Psychiatr Belg. 1976 Jul-Aug;76(4):658–666. [PubMed] [Google Scholar]
- DUDLEY H. F., BOLING E. A., LEQUESNE L. P., MOORE F. D. Studies on antidiuresis in surgery: effects of anesthesia, surgery and posterior pituitary antidiuretic hormone on water metabolism in man. Ann Surg. 1954 Sep;140(3):354–367. doi: 10.1097/00000658-195409000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flear C. T., Bhattacharya S. S., Singh C. M. Solute and water exchanges between cells and extracellular fluids in health and disturbances after trauma. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):98–120. doi: 10.1177/014860718000400209. [DOI] [PubMed] [Google Scholar]
- Halmagyi D. F., Broell J., Gump F. E., Kinney J. M. Hypermetabolism in sepsis: correlation with fever and tachycardia. Surgery. 1974 May;75(5):763–770. [PubMed] [Google Scholar]
- Hinshaw D. B., Burger J. M. Protective effect of glutamine on endothelial cell ATP in oxidant injury. J Surg Res. 1990 Sep;49(3):222–227. doi: 10.1016/0022-4804(90)90123-j. [DOI] [PubMed] [Google Scholar]
- Hoffer E. C., Meador C. K., Simpson D. C. Correlation of whole-body impedance with total body water volume. J Appl Physiol. 1969 Oct;27(4):531–534. doi: 10.1152/jappl.1969.27.4.531. [DOI] [PubMed] [Google Scholar]
- Jain P., Khanna N. K. Evaluation of anti-inflammatory and analgesic properties of L-glutamine. Agents Actions. 1981 May;11(3):243–249. doi: 10.1007/BF01967621. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Salloum R. M., Kasper M., Plumley D. A., Dolson D. J., Hautamaki R. D., Mendenhall W. R., Bova F. C., Bland K. I., Copeland E. M., 3rd Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg. 1990 Aug;125(8):1040–1045. doi: 10.1001/archsurg.1990.01410200104017. [DOI] [PubMed] [Google Scholar]
- Kushner R. F., Schoeller D. A. Estimation of total body water by bioelectrical impedance analysis. Am J Clin Nutr. 1986 Sep;44(3):417–424. doi: 10.1093/ajcn/44.3.417. [DOI] [PubMed] [Google Scholar]
- LLAURADO J. G. Increased excretion of aldosterone immediately after operation. Lancet. 1955 Jun 25;268(6878):1295–1298. doi: 10.1016/s0140-6736(55)92058-4. [DOI] [PubMed] [Google Scholar]
- Lacey J. M., Wilmore D. W. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990 Aug;48(8):297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Li S. J., Nussbaum M. S., McFadden D. W., Zhang F. S., LaFrance R. J., Dayal R., Fischer J. E. Addition of L-glutamine to total parenteral nutrition and its effects on portal insulin and glucagon and the development of hepatic steatosis in rats. J Surg Res. 1990 May;48(5):421–426. doi: 10.1016/0022-4804(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Lowe D. K., Benfell K., Smith R. J., Jacobs D. O., Murawski B., Ziegler T. R., Wilmore D. W. Safety of glutamine-enriched parenteral nutrient solutions in humans. Am J Clin Nutr. 1990 Dec;52(6):1101–1106. doi: 10.1093/ajcn/52.6.1101. [DOI] [PubMed] [Google Scholar]
- Lukaski H. C., Johnson P. E., Bolonchuk W. W., Lykken G. I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985 Apr;41(4):810–817. doi: 10.1093/ajcn/41.4.810. [DOI] [PubMed] [Google Scholar]
- McDonald G. B., Shulman H. M., Sullivan K. M., Spencer G. D. Intestinal and hepatic complications of human bone marrow transplantation. Part I. Gastroenterology. 1986 Feb;90(2):460–477. doi: 10.1016/0016-5085(86)90949-2. [DOI] [PubMed] [Google Scholar]
- Miller M. E., Cosgriff J. M., Forbes G. B. Bromide space determination using anion-exchange chromatography for measurement of bromide. Am J Clin Nutr. 1989 Jul;50(1):168–171. doi: 10.1093/ajcn/50.1.168. [DOI] [PubMed] [Google Scholar]
- Navari R. M., Buckner C. D., Clift R. A., Storb R., Sanders J. E., Stewart P., Sullivan K. M., Williams B., Counts G. W., Meyers J. D. Prophylaxis of infection in patients with aplastic anemia receiving allogeneic marrow transplants. Am J Med. 1984 Apr;76(4):564–572. doi: 10.1016/0002-9343(84)90274-2. [DOI] [PubMed] [Google Scholar]
- O'Dwyer S. T., Smith R. J., Hwang T. L., Wilmore D. W. Maintenance of small bowel mucosa with glutamine-enriched parenteral nutrition. JPEN J Parenter Enteral Nutr. 1989 Nov-Dec;13(6):579–585. doi: 10.1177/0148607189013006579. [DOI] [PubMed] [Google Scholar]
- Parry-Billings M., Evans J., Calder P. C., Newsholme E. A. Does glutamine contribute to immunosuppression after major burns? Lancet. 1990 Sep 1;336(8714):523–525. doi: 10.1016/0140-6736(90)92083-t. [DOI] [PubMed] [Google Scholar]
- SCHLOERB P. R., FRIIS-HANSEN B. J., EDELMAN I. S., SOLOMON A. K., MOORE F. D. The measurement of total body water in the human subject by deuterium oxide dilution; with a consideration of the dynamics of deuterium distribution. J Clin Invest. 1950 Oct;29(10):1296–1310. doi: 10.1172/JCI102366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeller D. A., van Santen E., Peterson D. W., Dietz W., Jaspan J., Klein P. D. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr. 1980 Dec;33(12):2686–2693. doi: 10.1093/ajcn/33.12.2686. [DOI] [PubMed] [Google Scholar]
- Stehle P., Zander J., Mertes N., Albers S., Puchstein C., Lawin P., Fürst P. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989 Feb 4;1(8632):231–233. doi: 10.1016/s0140-6736(89)91254-3. [DOI] [PubMed] [Google Scholar]
- Stein H. J., Hinder R. A., Oosthuizen M. M. Gastric mucosal injury caused by hemorrhagic shock and reperfusion: protective role of the antioxidant glutathione. Surgery. 1990 Aug;108(2):467–474. [PubMed] [Google Scholar]
- Szeluga D. J., Stuart R. K., Brookmeyer R., Utermohlen V., Santos G. W. Energy requirements of parenterally fed bone marrow transplant recipients. JPEN J Parenter Enteral Nutr. 1985 Mar-Apr;9(2):139–143. doi: 10.1177/0148607185009002139. [DOI] [PubMed] [Google Scholar]
- Wong W. W., Lee L. S., Klein P. D. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr. 1987 May;45(5):905–913. doi: 10.1093/ajcn/45.5.905. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R., Benfell K., Smith R. J., Young L. S., Brown E., Ferrari-Baliviera E., Lowe D. K., Wilmore D. W. Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):137S–146S. doi: 10.1177/0148607190014004201. [DOI] [PubMed] [Google Scholar]
- van Gelder N. M. Brain taurine content as a function of cerebral metabolic rate: osmotic regulation of glucose derived water production. Neurochem Res. 1989 Jun;14(6):495–497. doi: 10.1007/BF00964908. [DOI] [PubMed] [Google Scholar]


