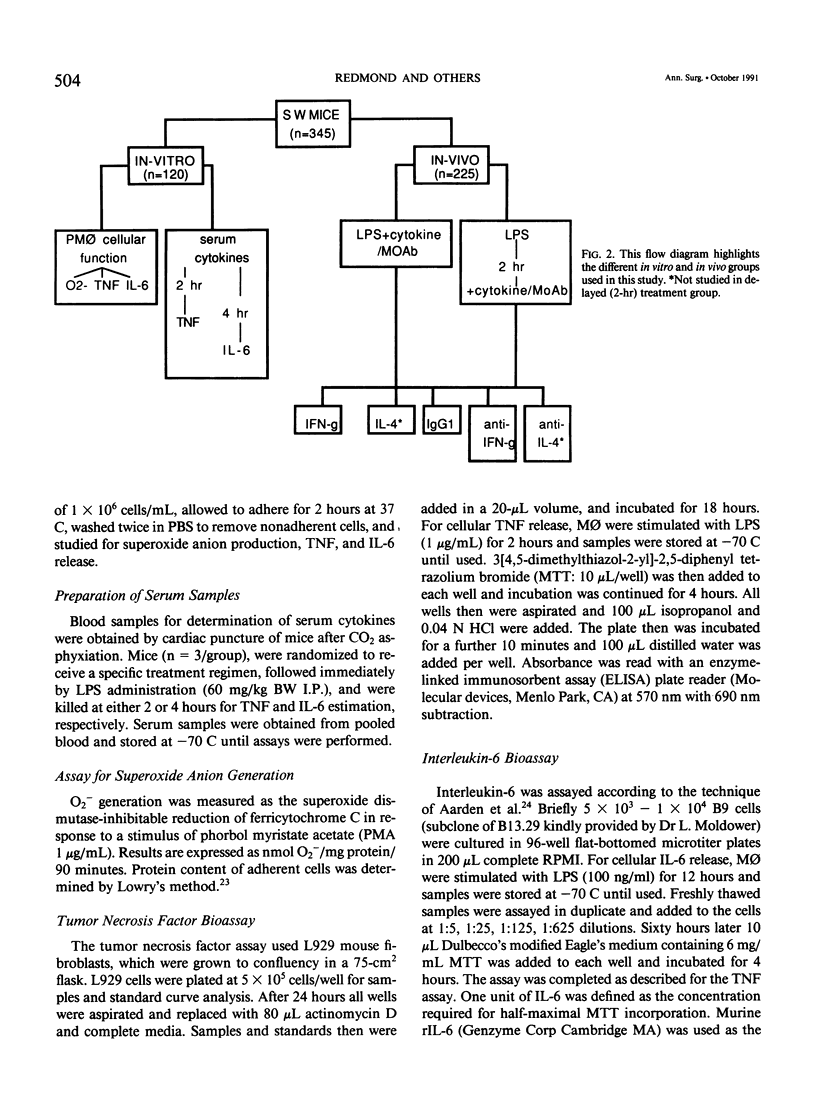
Abstract
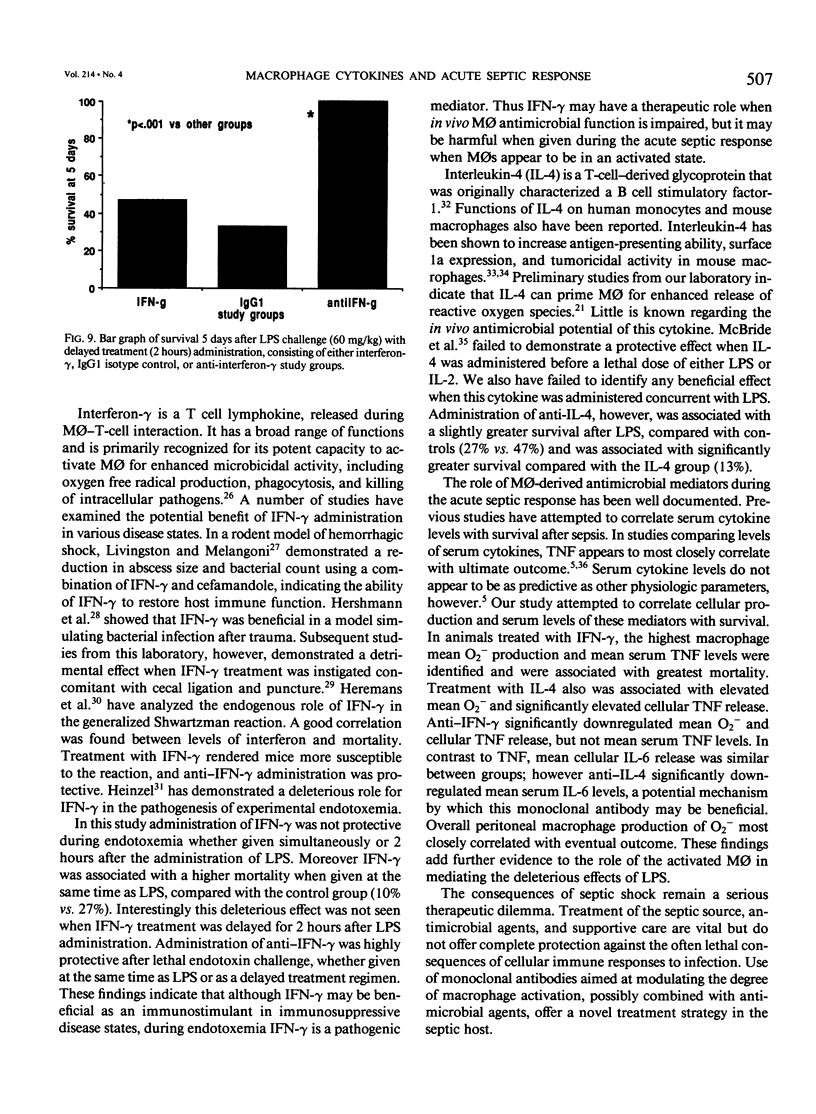
Interferon-gamma and other cytokines enhance macrophage (M phi) antimicrobial function and have been considered for therapeutic use in sepsis. Systemic sequelae of macrophage activation, however, are unclear. This study examined the effects of M phi activating cytokines (interferon-gamma [IFN-gamma] and interleukin-4 [IL-4]) and monoclonal antibodies directed against these cytokines in modulating the acute septic response. CFW/Swiss Webster mice (n = 345) received endotoxin (lipopolysaccharide [LPS]: 60 mg/kg body weight intraperitoneally) and were randomized to five treatment groups: IFN-gamma (10(4) units), IL-4 (10(4) units), IgG1 isotype antibody (TRFK5: 200 micrograms), anti-IFN-gamma (200 micrograms), or anti-IL-4 (200 micrograms) monoclonal antibodies (MAbs) given simultaneously or 2 hours after LPS. Animals were divided into two groups and studied for mortality or measurement of peritoneal M phi superoxide anion release (O2-), tumor necrosis factor (TNF), and IL-6 production 6 hours after administration of LPS +/- experimental regimens. Serum TNF and IL-6 also were assessed at 2 and 4 hours after LPS, respectively. Administration of LPS resulted in a 27% survival compared with 10% in the IFN-gamma and 13% in the IL-4 groups. Treatment with anti-IFN-gamma offered protection against LPS lethality (93%-100% survival, p less than 0.001 vs. other groups) when given either simultaneously or 2 hours after LPS. Anti-IFN-gamma also significantly decreased PM phi O-2 and TNF release. Thus anti-IFN-gamma may have an important role in the modulation of the acute septic response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brenner E. R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983 Jul-Aug;5(4):629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- Calandra T., Glauser M. P. Cytokines and septic shock. Diagn Microbiol Infect Dis. 1990 Sep-Oct;13(5):377–381. doi: 10.1016/0732-8893(90)90006-h. [DOI] [PubMed] [Google Scholar]
- Calandra T., Glauser M. P., Schellekens J., Verhoef J. Treatment of gram-negative septic shock with human IgG antibody to Escherichia coli J5: a prospective, double-blind, randomized trial. J Infect Dis. 1988 Aug;158(2):312–319. doi: 10.1093/infdis/158.2.312. [DOI] [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990 Nov 1;145(9):2920–2924. [PubMed] [Google Scholar]
- Heremans H., Van Damme J., Dillen C., Dijkmans R., Billiau A. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990 Jun 1;171(6):1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman M. J., Polk H. C., Jr, Pietsch J. D., Shields R. E., Wellhausen S. R., Sonnenfeld G. Modulation of infection by gamma interferon treatment following trauma. Infect Immun. 1988 Sep;56(9):2412–2416. doi: 10.1128/iai.56.9.2412-2416.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., Carling P. C., McCabe W. R. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med. 1980 Mar;68(3):332–343. doi: 10.1016/0002-9343(80)90101-1. [DOI] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., McCabe W. R. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980 Mar;68(3):344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livingston D. H., Malangoni M. A. Interferon-gamma restores immune competence after hemorrhagic shock. J Surg Res. 1988 Jul;45(1):37–43. doi: 10.1016/0022-4804(88)90018-2. [DOI] [PubMed] [Google Scholar]
- Mayoral J. L., Schweich C. J., Dunn D. L. Decreased tumor necrosis factor production during the initial stages of infection correlates with survival during murine gram-negative sepsis. Arch Surg. 1990 Jan;125(1):24–28. doi: 10.1001/archsurg.1990.01410130026003. [DOI] [PubMed] [Google Scholar]
- McBride W. H., Economou J. S., Nayersina R., Comora S., Essner R. Influences of interleukins 2 and 4 on tumor necrosis factor production by murine mononuclear phagocytes. Cancer Res. 1990 May 15;50(10):2949–2952. [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Natanson C., Eichenholz P. W., Danner R. L., Eichacker P. Q., Hoffman W. D., Kuo G. C., Banks S. M., MacVittie T. J., Parrillo J. E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989 Mar 1;169(3):823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Phillips W. A., Croatto M., Hamilton J. A. Priming the macrophage respiratory burst with IL-4: enhancement with TNF-alpha but inhibition by IFN-gamma. Immunology. 1990 Aug;70(4):498–503. [PMC free article] [PubMed] [Google Scholar]
- Silva A. T., Appelmelk B. J., Buurman W. A., Bayston K. F., Cohen J. Monoclonal antibody to endotoxin core protects mice from Escherichia coli sepsis by a mechanism independent of tumor necrosis factor and interleukin-6. J Infect Dis. 1990 Aug;162(2):454–459. doi: 10.1093/infdis/162.2.454. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Stuart P. M., Zlotnik A., Woodward J. G. Induction of class I and class II MHC antigen expression on murine bone marrow-derived macrophages by IL-4 (B cell stimulatory factor 1). J Immunol. 1988 Mar 1;140(5):1542–1547. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Espevik T. Interleukin 1 potentiates the lethal effect of tumor necrosis factor alpha/cachectin in mice. J Exp Med. 1988 Jun 1;167(6):1987–1992. doi: 10.1084/jem.167.6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Burke J. F., Thompson R. C., Dinarello C. A. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991 Mar 1;5(3):338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]


