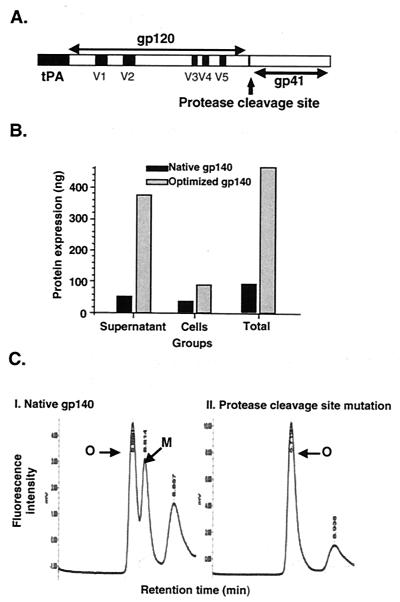
FIG. 1.
Structure, expression, and stabilization of HIV-1 US4 envelope glycoprotein in the oligomeric conformation. (A) Linear map of the HIV-1 gp120 and gp41 envelope glycoproteins. The gp120 variable regions (V1 to V5) are indicated as solid squares, and the mutation of arginine 509 to serine 509 in the protease cleavage site between gp120 and gp41 is indicated by an arrow. The gp41 portion includes the N and C α-helical regions known as oligomerization domains. tPA, human tissue-type plasminogen activator. (B) Effect of codon optimization on expression of o-gp140. Total protein expression obtained from native and codon-optimized constructs by using capture ELISA in different fractions is presented. (C) Effect of protease cleavage site mutation upon oligomer stabilization. 293 cells were transfected with gp140 protease cleavage site-mutated and nonmutated constructs by using the LT-1 transfection reagent as described for CHO cells in Materials and Methods. Supernatants were collected on day 5, and partial purification of the proteins was performed. These partially purified proteins were tested in a CD4 binding assay using a Bio Sil SEC-250 sizing column to separate monomers from oligomers as described in Materials and Methods. Peaks representing oligomer (O), monomer (M), and CD4 are indicated.