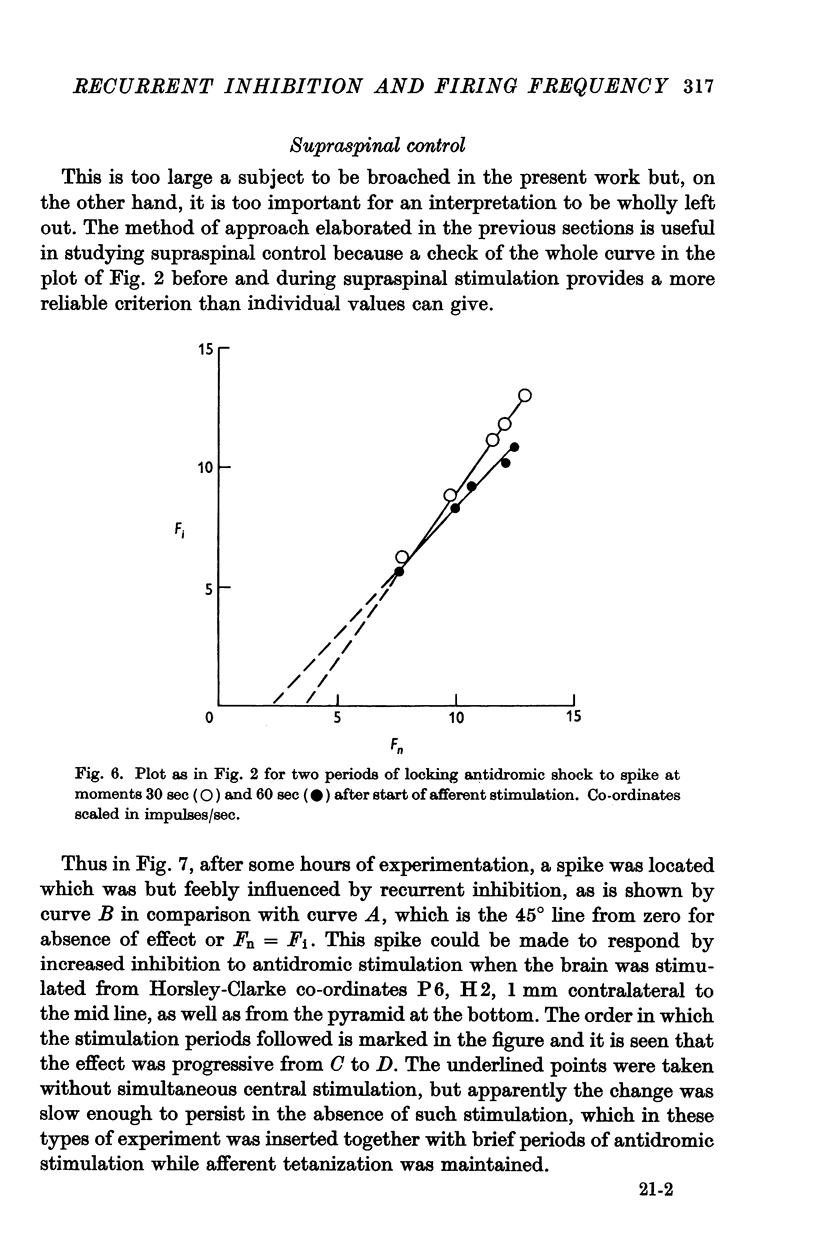
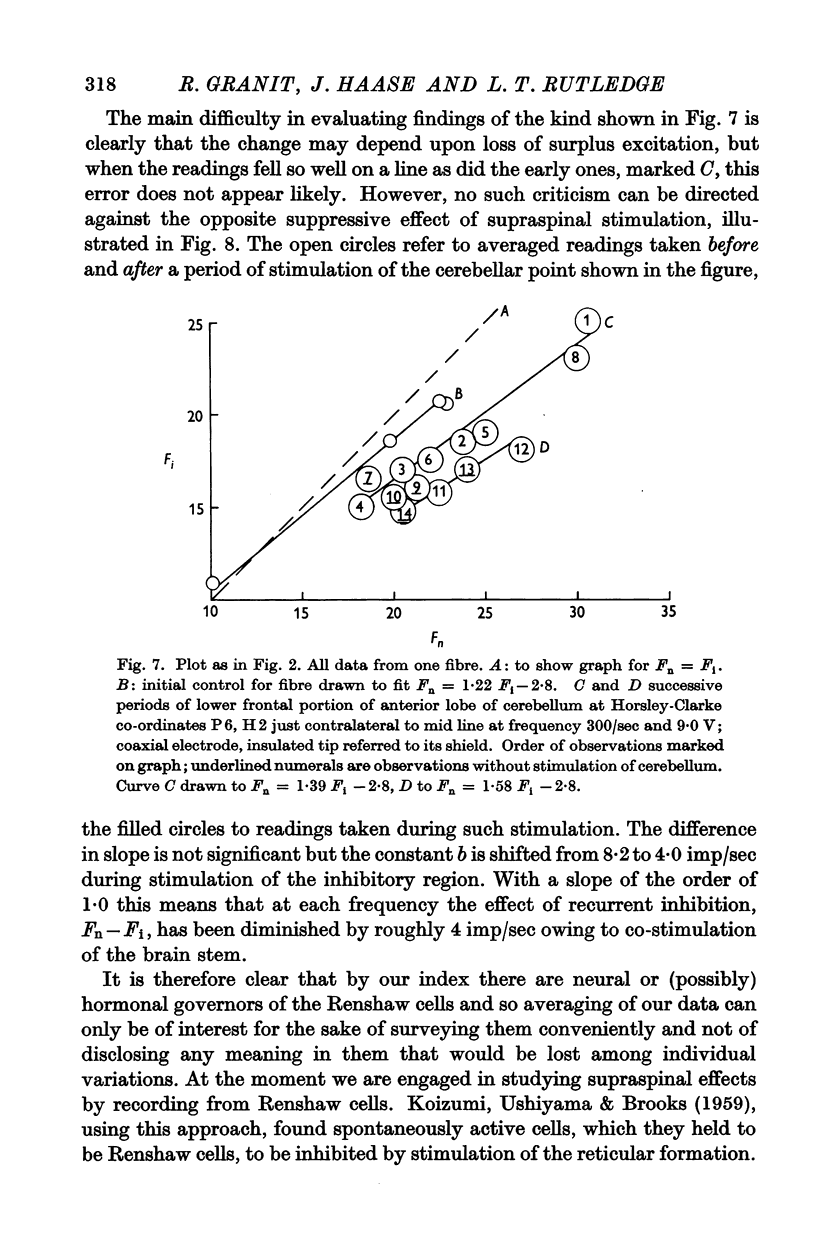
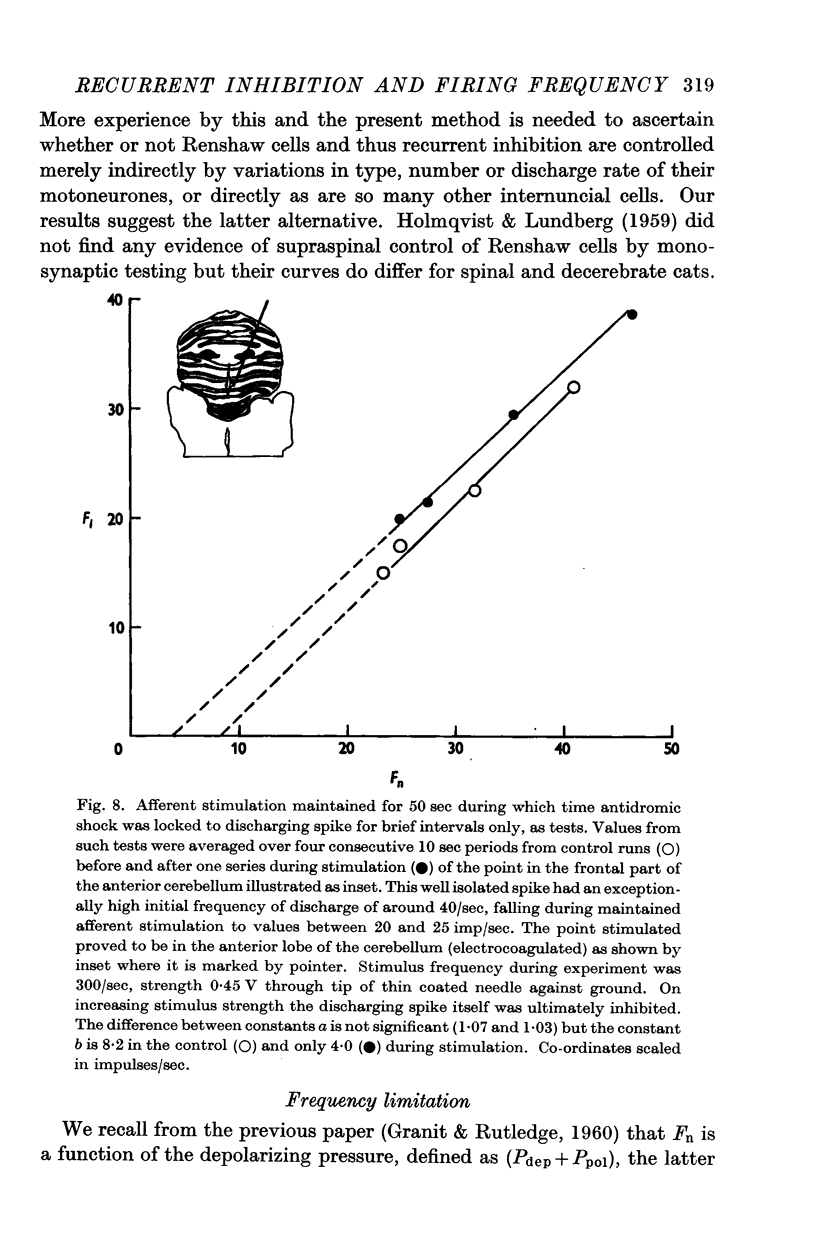
Full text
PDF



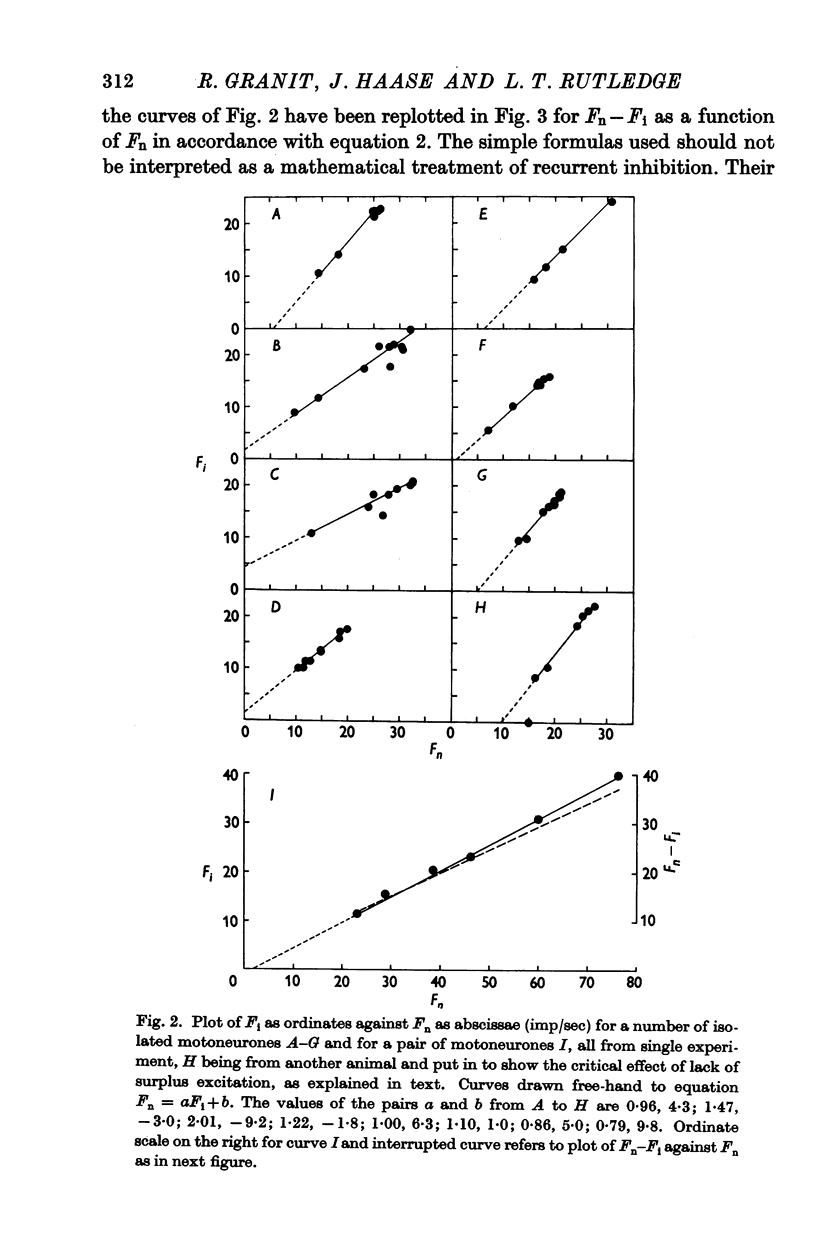
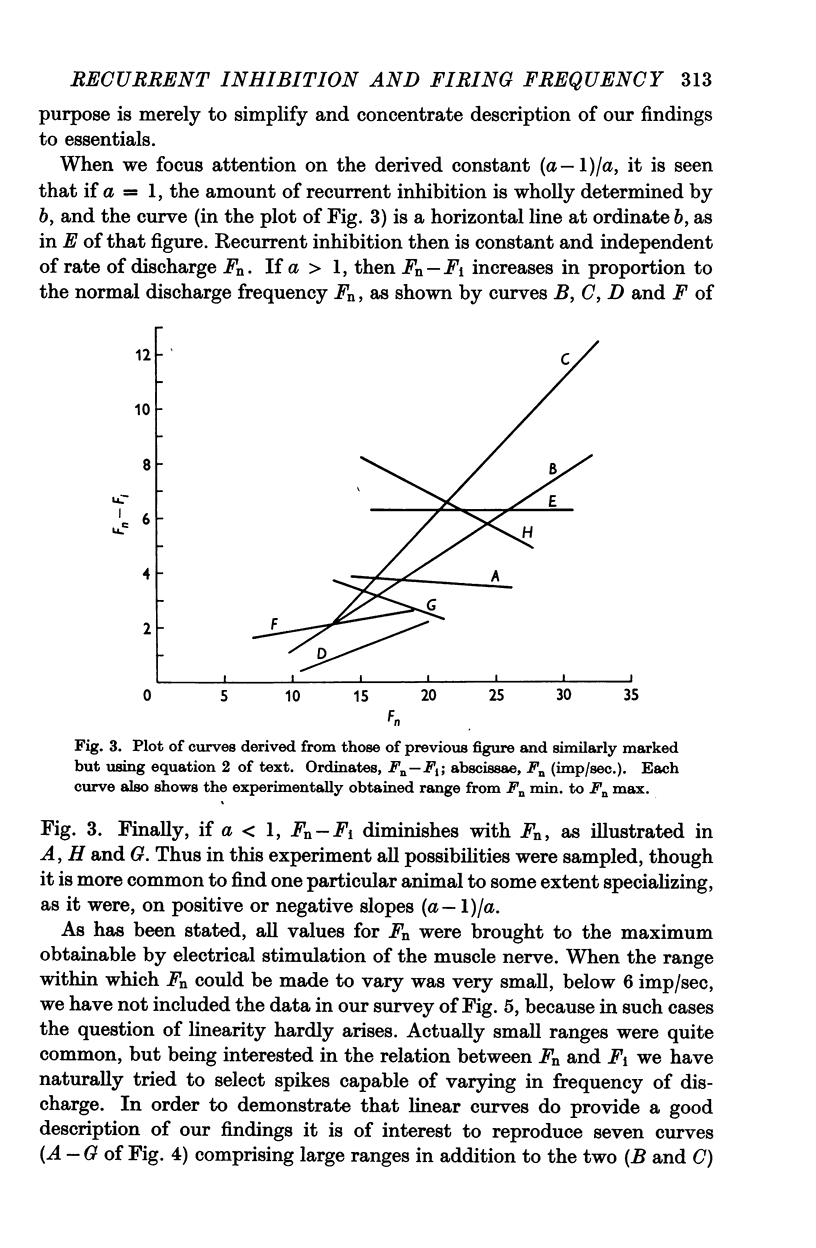
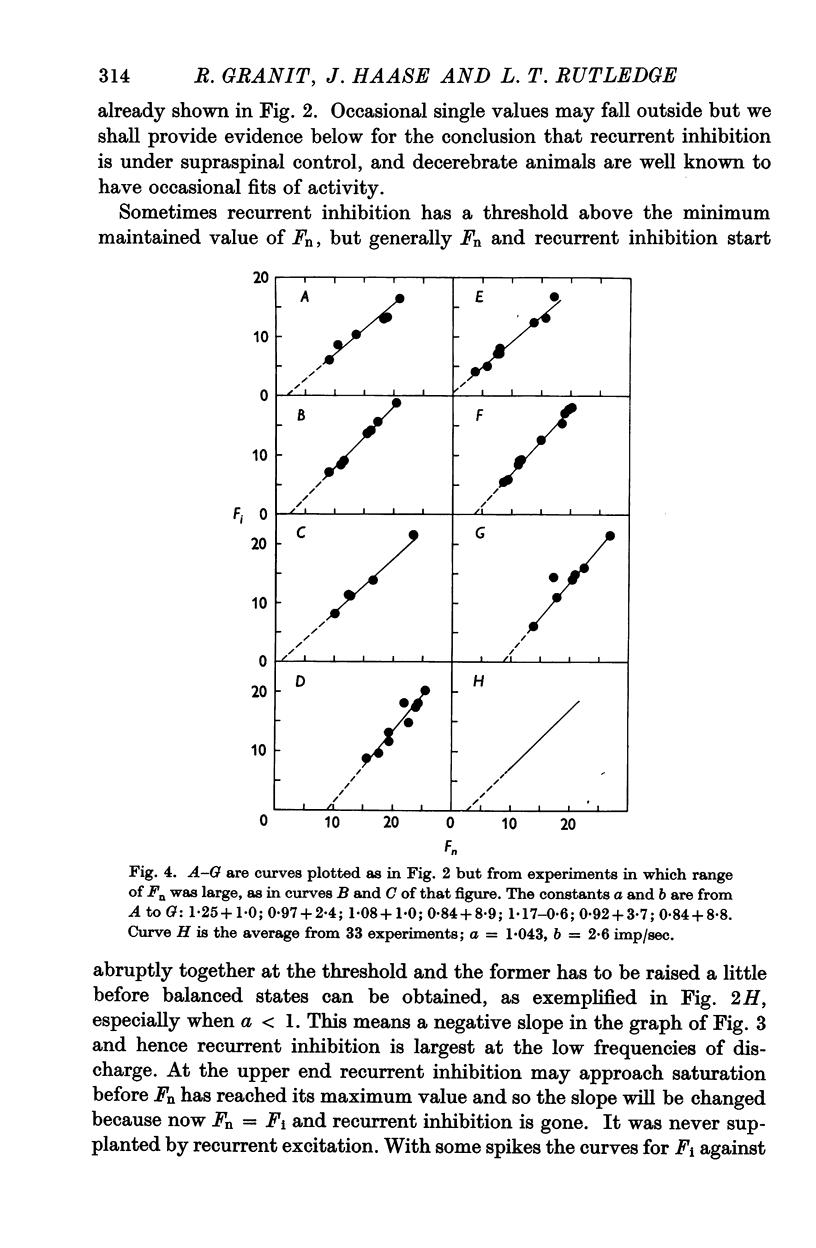
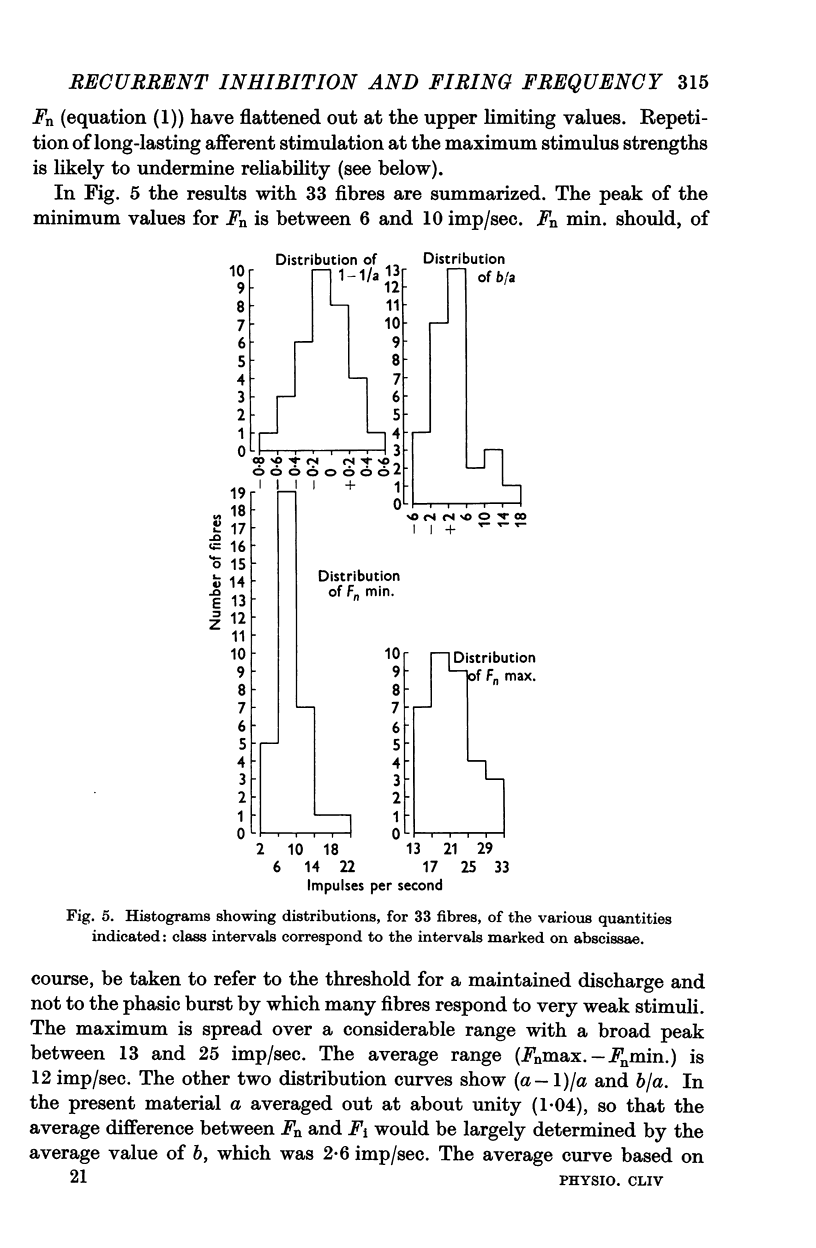










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVORD E. C., Jr, FUORTES M. G. Reflex activity of extensor motor units following muscular afferent excitation. J Physiol. 1953 Nov 28;122(2):302–321. doi: 10.1113/jphysiol.1953.sp005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAKI T., OTANI T. Accommodation and local response in motoneurons of toad's spinal cord. Jpn J Physiol. 1959 Mar 25;9(1):69–83. doi: 10.2170/jjphysiol.9.69. [DOI] [PubMed] [Google Scholar]
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- Adrian E. D., Bronk D. W. The discharge of impulses in motor nerve fibres: Part II. The frequency of discharge in reflex and voluntary contractions. J Physiol. 1929 Mar 20;67(2):i3–151. [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The generation of impulses in motoneurones. J Physiol. 1957 Dec 3;139(2):232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The interpretation of spike potentials of motoneurones. J Physiol. 1957 Dec 3;139(2):198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., FRANK K., BECKER M. C. Steps in the production of motoneuron spikes. J Gen Physiol. 1957 May 20;40(5):735–752. doi: 10.1085/jgp.40.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D., STEG G. Tonic and phasic ventral horn cells differentiated by post-tetanic potentiation in cat extensors. Acta Physiol Scand. 1956 Sep 26;37(2-3):114–126. doi: 10.1111/j.1748-1716.1956.tb01347.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol. 1958 Oct 31;143(3):387–402. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PASCOE J. E., STEG G. The behaviour of tonic alpha and gamma motoneurones during stimulation of recurrent collaterals. J Physiol. 1957 Oct 30;138(3):381–400. doi: 10.1113/jphysiol.1957.sp005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- GRANIT R., RUTLEDGE L. T. Surplus excitation in reflex action of motoneurones as measured by recurrent inhibition. J Physiol. 1960 Dec;154:288–307. doi: 10.1113/jphysiol.1960.sp006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., SKOGLUND S., THESLEFF S. Activation of muscle spindles by succinylcholine and decamethonium, the effects of curare. Acta Physiol Scand. 1953;28(2-3):134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- HARTLINE H. K., RATLIFF F. Inhibitory interaction of receptor units in the eye of Limulus. J Gen Physiol. 1957 Jan 20;40(3):357–376. doi: 10.1085/jgp.40.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENATSCH H. D., SCHULTE F. J. Reflexerregung und Eigenhemmung tonischer und phasischer Alpha-Motoneurone während chemischer Dauererregung der Muskelspindeln. Pflugers Arch. 1958;268(2):134–147. doi: 10.1007/BF00386085. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E. Relation between size of neurons and their susceptibility to discharge. Science. 1957 Dec 27;126(3287):1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- HOLMGREN B., MERTON P. A. Local feedback control of motoneurones. J Physiol. 1954 Feb 26;123(2):47–8P. [PubMed] [Google Scholar]
- JOB C. Uber autogene Inhibition und Reflexumkehr bei spinalisierten und decerebrierten Katzen. Pflugers Arch. 1953;256(5):407–418. [PubMed] [Google Scholar]
- KOIZUMI K., USHIYAMA J., BROOKS C. M. A study of reticular formation action on spinal interneurons and motoneurons. Jpn J Physiol. 1959 Sep 15;9:282–303. doi: 10.2170/jjphysiol.9.282. [DOI] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. C. Post-tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J Gen Physiol. 1949 Nov;33(2):147–170. doi: 10.1085/jgp.33.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. Monosynaptic reflex response of individual motoneurons as a function of frequency. J Gen Physiol. 1957 Jan 20;40(3):435–450. doi: 10.1085/jgp.40.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. A study of certain factors influencing the stretch reflex of the decerebrate cat. J Physiol. 1959 Oct;147(3):547–564. doi: 10.1113/jphysiol.1959.sp006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The dependence of tension upon extension in the stretch reflex of the soleus muscle of the decerebrate cat. J Physiol. 1959 Oct;147(3):521–546. doi: 10.1113/jphysiol.1959.sp006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS C. G. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):1–25. doi: 10.1113/expphysiol.1959.sp001364. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Motor end-plate 'desensitization' by repetitive nerve stimuli. J Physiol. 1959 Oct;148:659–664. doi: 10.1113/jphysiol.1959.sp006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON V. J. Recurrent facilitation of spinal reflexes. J Gen Physiol. 1959 Mar 20;42(4):703–713. doi: 10.1085/jgp.42.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., DIECKE F. P. Distribution of recurrent facilitation and inhibition in cat spinal cord. J Neurophysiol. 1960 Mar;23:144–153. doi: 10.1152/jn.1960.23.2.144. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., DIECKE F. P. Pattern of recurrent conditioning of spinal reflexes. Nature. 1959 Mar 21;183(4664):824–825. doi: 10.1038/183824a0. [DOI] [PubMed] [Google Scholar]


