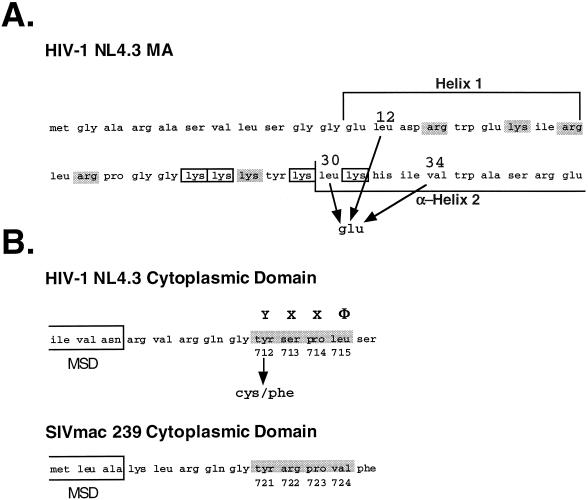
FIG. 1.
Locations of mutations in the HIV-1 (NL4-3) MA and Env domains. (A) Mutations in the MA protein that confer a block to infectivity. The numbers indicate the positions of the mutations subsequent to cleavage of the N-terminal Met residue. Charged residues, identified as essential for MA function, are indicated by boxes, whereas those residues which are mutable without consequence are shaded. Designation of the helices is according to the crystal structure determined by Hill et al. (23). (B) The membrane-spanning region (indicated by the boxes) and the immediately distal cytoplasmic domain sequences for the Env proteins of HIV-1 (NL4-3) and SIVmac 239 are aligned. The region predicted to form a consensus YXXΦ endocytosis signal is identified by shading. The numbering of amino acid residues is from the initiation of the Env precursor.