Fig. 6. Functional p38 MAPK activity is required for TGF-β1-induced PKB/Akt phosphorylation.
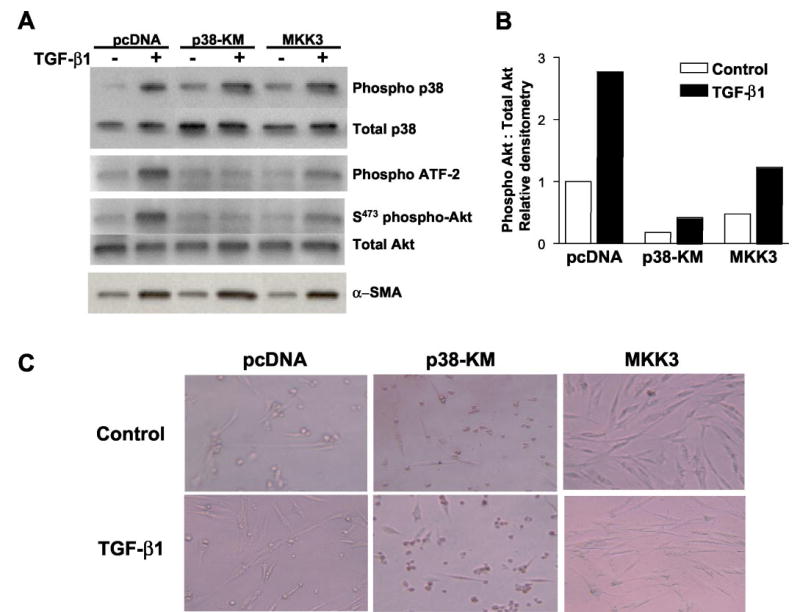
A, IMR-90 cells stably transfected with control plasmid (pcDNA), a kinase-deficient p38 MAPK construct (p38-KM), or wild-type MKK3 (MKK3) were grown to near-confluence, growth-arrested for 48 h prior to treatment with or without TGF-β1 (2 ng/ml), and cell lysates obtained at 1, 12, and 16 h. Phosphorylation of p38 MAPK was assessed at 1 h following TGF-β1 treatment by Western immunoblotting with antibodies to phospho- and total p38 MAPK (top panels). p38 MAP kinase activity was assessed at 1 h following TGF-β1 treatment by in vitro phosphorylation of the p38 MAPK substrate, ATF-2, as described under “Materials and Methods” (middle top panel). Phosphorylation of PKB/Akt was assessed at 12 h following TGF-β1 treatment by Western immunoblotting with antibodies to phospho- and total Akt (middle bottom panels). Cell lysates obtained 16 h after treatment with TGF-β1 were subjected to Western immunoblotting for α-smooth muscle actin (α-SMA), a marker of myofibroblast differentiation (bottom panel). B, densitometric analyses of the phospho-Akt:total Akt blots represented in A. C, stably transfected cells were cultured to 80% confluence, growth-arrested in DMEM with 0.01% FBS for 48 h, treated with/without TGF-β1 (2 ng/ml) for 2 days. Representative photographs demonstrate cellular morphology under these serum-deprived conditions.
