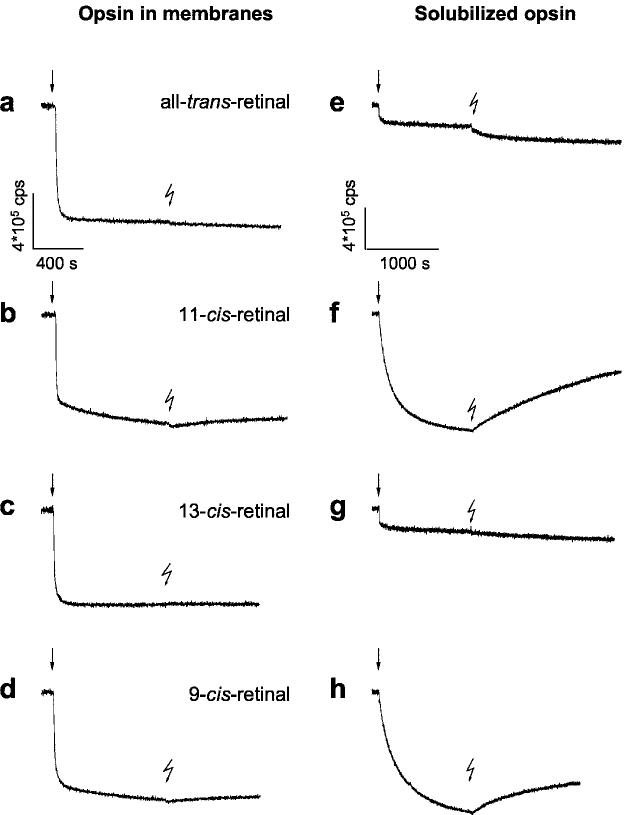
Fig. 2.
Time-dependent fluorescence changes in opsin apoprotein evoked by retinal isomers. Changes in intrinsic protein fluorescence were recorded upon addition of retinals (1 μm) to opsin (1 μm) at 20 °C (pH 6.5). Fluorescence emission of opsin in native membrane (left, a–d) and in 0.1% n-dodecyl-β-maltoside (right, e–h) was recorded at 330 nm with λex = 295 nm. The rapid initial quenching by retinal addition (arrows) is termed the uptake signal. Each sample was illuminated for 15 s with green light (OG 495) after incubation, as indicated by the flash symbol. The fluorescence change after illumination was assigned to the release of the photoisomerized chromophore during decay of the activated metarhodopsin II (8, 9)