Abstract
Staphylococcus aureus is widely prevalent in the nasopharynges of healthy individuals (carriers) but can also cause serious infections. S. aureus can elaborate a variety of superantigen exotoxins in “carrier” or “pathogenic” states. Streptococcus pyogenes can also colonize the nasopharynx and elaborate superantigens. Unlike the acute effects of superantigen exotoxins absorbed through the gut or vaginal mucosa, little is known regarding the pathogenesis of superantigens entering through the intranasal route. In the current study, we evaluated the local and systemic effects of staphylococcal enterotoxin B (SEB) and streptococcal pyrogenic exotoxin A (SPEA) delivered through the intranasal route. Superantigens were administered intranasally on multiple occasions, and experimental animals were sacrificed on day 8 for experimental analyses. SEB-induced airway inflammation was more pronounced for HLA-DR3 transgenic mice than for BALB/c mice, consistent with bacterial superantigens binding more efficiently to human than murine major histocompatibility complex class II. The nature of the airway inflammation in HLA-DR3 mice was determined by the concentration of SEB applied intranasally. Low concentrations (20 ng) induced eosinophilic airway inflammation as well as eosinophil degranulation, whereas intranasal exposure to higher concentrations (2,000 ng) resulted in neutrophilic airway inflammation, permanent airway destruction, toxic shock, and mortality. SEB-induced eosinophilic inflammatory response was enhanced in signal transducer and activator of transcription (STAT)-4-deficient HLA-DQ8 transgenic mice with defective interleukin-12 signaling. Intranasal administration of SPEA induced airway inflammation and systemic immune activation in HLA-DQ8 transgenic mice. In conclusion, repeated chronic intranasal exposure to bacterial superantigens causes airway inflammation and systemic immune activation.
Bacterial superantigens are a family of microbial polypeptide exotoxins capable of inducing strong proliferation of T lymphocytes even at extremely low concentrations (21, 63). They are the most potent T-cell mitogens known to humankind. Mechanistically, unlike conventional antigens which bind to the peptide-binding groove of major histocompatibility complex (MHC) class II molecules after undergoing a series of processing steps, superantigens bind directly to MHC class II molecules outside of the peptide-binding groove without undergoing processing. While exogenous antigens presented by MHC class II molecules activate CD4+ T cells (34), MHC class II-bound superantigens activate both CD4+ and CD8+ T cells. While conventional antigens activate specific T cells by interacting with both α and β chains of T-cell receptor (TCR) molecules (83), superantigens activate T cells by binding directly to the variable region of the TCR β chain (and in rare cases TCR Vα families [69]), independent of their antigen specificities (56). Superantigens thus activate 30 to 50% of the total T-cell pool, whereas conventional antigens activate approximately 1 in 104 to 1 in 106 T cells (56). By virtue of their capacity to induce strong immune activation, superantigens can cause a variety of illnesses, ranging from acute food poisoning to toxic shock syndrome (21, 51), and have even been implicated in some autoimmune diseases (71, 108).
Bacterial superantigens are produced primarily by Staphylococcus aureus and Streptococcus pyogenes (71). S. aureus is ubiquitous in nature (7, 12, 58), and humans are natural carriers (primarily the nasal passage) (12, 68). While more than half of the pathogenic isolates produce one or more superantigen exotoxins (6, 28, 104), even strains isolated from asymptomatic carriers can produce superantigens (6, 28, 35, 49, 64, 103). The nasopharynx is also the site of colonization by group A, group C, and group G streptococci. Among these, S. pyogenes is considered the most pathogenic and elaborates a variety of superantigen exotoxins (77). Pathogenic S. pyogenes strains producing pyrogenic exotoxins have been isolated from asymptomatic schoolchildren living in areas with reported outbreaks of invasive streptococcal disease (15). While group C and group G streptococci are generally considered commensals, recent studies have demonstrated that they too can harbor genes encoding superantigen exotoxins (41, 45, 85). In addition to the presence of genes encoding bacterial superantigens in these isolates, recent studies have demonstrated the presence of transcripts encoding bacterial superantigens following experimental infection (102) and exotoxins themselves in patients with staphylococcal nasal carriage (89).
Given the presence of S. aureus and group A, group C, and group G streptococci in the human nasopharynx in either the carrier state or the disease state, it is probable that the respiratory tract is exposed to their products, including superantigens (3, 31, 40, 48, 70). It has been shown that staphylococcal protein A (30), cell wall peptidoglycan and lipoteichoic acid (54), and bacterial DNA containing unmethylated CpG motifs (88) can cause inflammation of the airways and lungs following direct exposure through the airway. However, the pathological effects of superantigen exotoxins produced by S. aureus and S. pyogenes on the respiratory system following direct exposure through the nasopharyngeal route have not been explored. As bacterial superantigens are potent T-cell mitogens, they have the potential to cause airway and lung inflammation through the same mechanisms by which they cause other diseases. While there are some indications that bacterial superantigens play a role in airway inflammation, including with asthma (4, 36, 37, 79) and chronic obstructive pulmonary disease (80), a definite role for bacterial superantigens in airway and/or lung inflammation has not yet been established using an appropriate animal model. Not only does this have immense clinical relevance, but the looming threat of the deployment of aerosolized superantigens as biological weapons necessitates elucidation of the effects of superantigen delivered directly to the nasal passage (33, 44, 60). In the current study, we evaluated the concentration-dependent effects of bacterial superantigen on the airways and lungs following intranasal exposure using a mouse model. Since bacterial superantigens bind poorly to murine MHC class II molecules (52), we used mice lacking endogenous class II but transgenically expressing high-affinity human HLA class II molecules (16, 73-76, 94, 110). Results of this study offer novel insight into the pathogenesis of airway inflammation.
MATERIALS AND METHODS
Mice.
HLA-DR3 transgenic mice expressing functional HLA-DRA1*0101 and HLA-DRB1*0301 transgenes on the complete mouse MHC class II-deficient background (AEo) (14) have been previously described (74). Previously described HLA-DQ8 transgenic mice expressing HLA-DQA1*0301 and HLA-DQB1*0302 transgenes (74) were also studied. Signal transducer and activator of transcription (STAT)-4-deficient, HLA-DQ8 transgenic mice were generated by William Stohl (University of Southern California Keck School of Medicine, Los Angeles, CA). T-cell responses in these HLA transgenic mice are restricted only by the transgenic HLA class II molecules (96). BALB/c mice were from The Jackson Laboratory (Bar Harbor, ME). Mice were bred within the barrier facility of Mayo Clinic Immunogenetics Mouse Colony (Rochester, MN) and moved to a conventional facility after weaning. All experiments were approved by the Institutional Animal Care and Use Committee.
Superantigen and animal challenge.
Staphylococcal enterotoxin B (SEB) and streptococcal pyrogenic exotoxin A (SPEA) (Toxin Laboratories, Sarasota, FL) were dissolved in sterile, endotoxin-free phosphate-buffered saline (PBS) and stored at −80°C. For intraperitoneal challenge, mice received the indicated concentrations of SEB in 200 μl of PBS. For intranasal challenge, mice were anesthetized with 2,2,2-tribromoethanol (Avertin; Aldrich Chemical, Milwaukee, WI), at a dose adjusted so that pedal reflexes were absent. Subsequently, mice were held in an upright position and 50 μl PBS alone or different concentrations of superantigens dissolved in 50 μl PBS were instilled into the nostrils. Mice were laid in a supine position until they recovered from anesthesia.
Measurement of AHR.
Airway hyperreactivity (AHR) was assessed on the eighth day after the first intranasal SEB application by methacholine-induced airflow obstruction in conscious mice with a whole-body plethysmograph (Buxco Electronics, Troy, NY), using a previously described procedure (50). Pulmonary airflow obstruction was measured by enhanced pause (Penh) as follows: Penh = (Te/RT − 1) × (PEF/PIF), where Penh is enhanced pause (dimensionless), Te is expiratory time, RT is relaxation time, PEF is peak expiratory flow (ml/s), and PIF is peak inspiratory flow (ml/s). Penh, minute volume, tidal volume, and breathing frequency were obtained from chamber pressure, measured with a transducer connected to preamplifier modules, and analyzed by system software (all from Buxco Electronics). To measure methacholine responsiveness, mice were exposed to PBS for 2 min, followed by an incremental dosage (3 to 50 mg/ml) of aerosolized methacholine (freshly prepared in PBS); Penh was monitored for each dose of methacholine. Results for PBS and methacholine were expressed as percentage of baseline Penh values before PBS exposure.
Collection of BAL fluid.
Immediately after AHR was measured, mice were injected intraperitoneally with a lethal dose of 2,2,2-tribromoethanol. The trachea was cannulated, the lungs were lavaged twice with 0.5 ml of PBS, and the fluids were pooled. After centrifugation, the supernatant was collected and stored at −80°C. The cells were resuspended and counted using a hemacytometer. Bronchoalveolar lavage (BAL) fluid cell differentials were determined by Wright-Giemsa staining; 200 cells were differentiated using conventional morphological criteria. Concentrations of gamma interferon (IFN-γ) in BAL fluid supernatants were measured using an enzyme-linked immunosorbent assay kit as directed by the manufacturer (R&D Systems, Minneapolis, MN).
Flow cytometry.
Mononuclear cells from lymphoid organs and BAL fluid were stained with fluorescein isothiocyanate-conjugated anti-Vβ6 or anti-Vβ8, phycoerythrin-conjugated CD4, and peridinin chlorophyll protein-conjugated CD8. Alternatively, B cells and macrophages were analyzed using B220 and Mac-1 antibodies, respectively. All antibodies were from BD Pharmingen (San Diego, CA).
Histopathology and immunohistochemistry.
After BAL fluid collection, the lungs were fixed in 10% formalin and embedded in paraffin. Six-millimeter sections were stained with hematoxylin and eosin for histopathological evaluation. For demonstrating eosinophils, processed formalin-fixed tissue sections were stained with rabbit anti-mouse major basic protein (MBP), as previously described (50).
Statistics.
Statistical evaluation was carried out using InStat (version 3.0; GraphPad Software, Inc., San Diego, CA). Student's t test or analysis of variance (ANOVA) was performed, depending on the number of groups in each experiment.
RESULTS
Response to SEB in HLA-DR3 transgenic mice versus response in BALB/c mice.
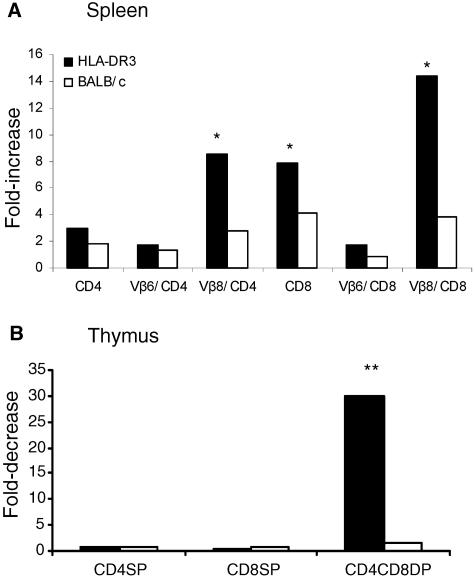
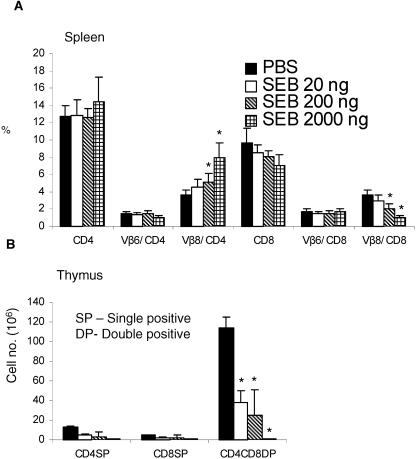
Intraperitoneal challenge with 10 μg of SEB resulted in a significantly higher (P < 0.05, unpaired Student's t test) expansion of CD4+ and CD8+ T cells bearing SEB-reactive TCR Vβ8, but not the nonreactive TCR Vβ6, in the spleens of HLA-DR3 transgenic mice than in spleens of BALB/c mice (Fig. 1A). Further, as depicted in Fig. 1B, the CD4 CD8 double-positive thymocytes in HLA-DR3 mice underwent a significant reduction compared to those in BALB/c mice (P < 0.001, unpaired Student's t test). Two injections of 10 μg SEB 48 h apart resulted in 100% mortality in HLA-DR3 mice (mortality, four of four mice), whereas all of the BALB/c mice tested survived this challenge (mortality, zero of four mice). These results confirm that HLA class II transgenic mice are better models for studying the immune response to superantigens than are conventional mouse strains.
FIG. 1.
T-cell responses following systemic SEB challenge. Age-matched BALB/c or HLA-DR3 transgenic mice were challenged with a single intraperitoneal injection of 10 μg of SEB. Mice were sacrificed 3 days later, and the distributions of different T-cell subsets in the spleen (A) or thymus (B) were enumerated by flow cytometry. Bars indicate ratios between absolute numbers of cells in PBS-treated and SEB-treated mice. Each bar represents the mean of four mice/group. *, P < 0.05; **, P < 0.001; SP, single positive; DP, double positive.
Intranasal delivery of SEB results in inflammation of the airways and lungs in HLA-DR3 transgenic mice.
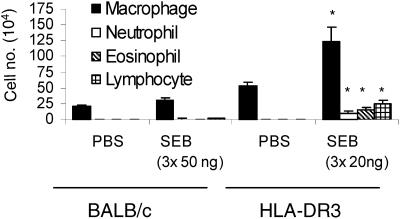
To compare the direct effects of SEB on the airways of HLA-DR3 and BALB/c mice, we challenged these two strains with the indicated concentrations of SEB by the intranasal route (Fig. 2). Intranasal delivery of SEB resulted in a significantly higher accumulation of macrophages, eosinophils, neutrophils, and lymphocytes in BAL fluid of HLA-DR3 mice than in BAL fluid of BALB/c mice (P < 0.05, unpaired Student's t test), even though the latter were challenged with 2.5 times the dose used for the former. Only alveolar macrophages were detected in BAL fluid of PBS-challenged mice from either group. Since SEB elicited more-severe airway inflammation in HLA-DR3 mice than in BALB/c mice, the former were used in subsequent studies.
FIG. 2.
Airway inflammation following intranasal SEB challenge. Age-matched BALB/c or HLA-DR3 transgenic mice were challenged intranasally with PBS or SEB (50 ng for BALB/c and 20 ng for HLA-DR3 transgenic mice) on days 0, 3, and 6. Mice were sacrificed on day 8, and leukocyte subsets in BAL fluid were enumerated with Wright-Giemsa-stained cytospin preparations. Each bar represents the mean ± standard error from 4 BALB/c or 10 HLA-DR3 mice. *, P < 0.05.
Concentration-dependent variations in airway responses to SEB in HLA-DR3 transgenic mice.
We evaluated the effect of SEB concentration (20, 200, or 2,000 ng) on airway inflammation by challenging HLA-DR3 mice with increasing concentrations of SEB and measuring airway responses on the eighth day. Mice administered 20 ng of SEB did not show any visible signs of distress and appeared healthy throughout the study. However, mice administered 2,000 ng of SEB exhibited clear signs of distress, including ruffled fur, labored breathing, listlessness, and weight loss. While the body weights of the mice in all groups were comparable on day 0, the body weights of the PBS group was 25.25 ± 1.70 g (n = 9) on day 8, while that of the SEB 2,000-ng group was 16.6 ± 1.7 g (n = 4) (P = 0.0002, unpaired Student's t test).
While no mortality was noted after the first dose of intranasal SEB delivery for any of the groups studied, high mortality was observed after the second or third dose of intranasal SEB delivery for the 200-ng (mortality, 4/10 mice) and 2,000-ng (mortality, 6/10 mice) groups. Mortality was not observed during or immediately after administration of SEB but rather occurred during the ensuing 24 to 48 h. Thus, airway exposure to low concentrations of SEB did not cause any apparent clinical signs, while higher concentrations of SEB caused signs consistent with toxic shock and were associated with mortality.
We evaluated AHR in mice that had been administered SEB or PBS intranasally by measuring the methacholine-induced airway changes at the termination of the experiment (i.e., on day 8). Mice that received PBS and 20 ng/dose of SEB had comparable baseline AHRs as measured by Penh. However, airway resistance in the SEB 20-ng group showed definite changes in response to increasing concentrations of inhaled methacholine compared to airway resistance in the PBS-treated group (P = 0.25, 0.045, 0.05, and 0.313 at 3, 6, 12, and 25 mg of methacholine, respectively; unpaired Student's t test) (Fig. 3A). On the other hand, mice that received 200 and 2,000 ng of SEB had significantly higher baseline AHRs than mice that received intranasal PBS (P < 0.05, ANOVA). AHR changed little following methacholine challenge in these groups. This indicated that while a low concentration of SEB (20 ng) was associated with reversible AHR, higher concentrations of SEB (200 and 2,000 ng) were associated with permanent airway changes and showed little methacholine-induced change (Fig. 3A), suggesting a concentration-dependent effect of SEB on certain airway responses.
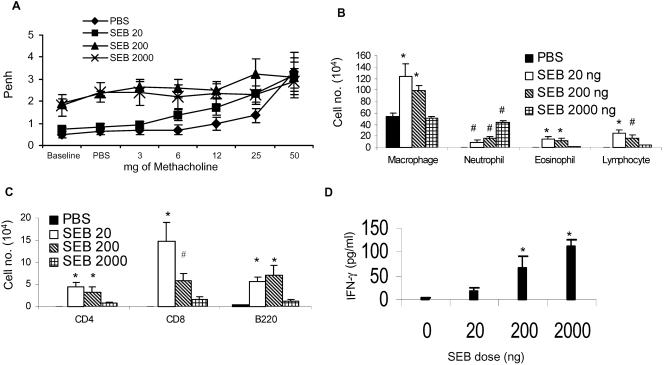
FIG. 3.
Concentration-dependent airway hyperresponsiveness and airway inflammation following intranasal SEB challenge. Age-matched HLA-DR3 transgenic mice were challenged intranasally with PBS or SEB (20, 200, or 2,000 ng) on days 0, 3, and 6. (A) Basal as well as methacholine-induced airway responsiveness was measured using a whole-body plethysmograph on day 8. (B) BAL fluid differentials. (C) Flow cytometric analysis of BAL fluids from mice from panel A. (D) Levels of IFN-γ in BAL fluid were measured by enzyme-linked immunosorbent assay. Each data point represents the mean ± standard error from 10 mice. *, P < 0.01; #, P < 0.05.
We next studied the effect of variation in SEB concentration on the types of leukocytes recruited to the airways by analyzing the BAL fluid (Fig. 3B). Lower concentrations of SEB recruited more eosinophils into BAL fluid than did higher concentrations of SEB (P < 0.01 for PBS versus SEB 20-ng groups; P < 0.05 for PBS versus SEB 200-ng groups as well as SEB 20-ng versus 2,000-ng groups; one-way ANOVA). Higher concentrations of SEB were associated with more neutrophil recruitment into BAL fluid than were lower concentrations (P < 0.05 for all SEB groups compared to the PBS group; P < 0.001 for SEB 2,000-ng versus 20-ng or 200-ng groups; one-way ANOVA). The eosinophil-to-neutrophil ratios were 1.6, 0.75, and 0.03 for 20, 200, and 2,000 ng/dose of intranasal SEB, respectively. Macrophages were more abundant at lower SEB concentrations (P < 0.01 for PBS versus SEB 20-ng groups as well as PBS versus SEB 200-ng groups; P < 0.05 for SEB 20-ng versus SEB 2,000-ng groups; one-way ANOVA). An inverse concentration effect was also seen for lymphocyte recruitment. Lymphocytes were more abundant at lower SEB concentrations (Fig. 3B and C) (P < 0.01 for PBS versus SEB 20-ng groups; P < 0.05 for PBS versus SEB 200-ng groups as well as SEB 20-ng versus SEB 2,000-ng groups; one-way ANOVA). Together, these results suggest that bacterial superantigens are capable of inducing concentration-dependent AHR and that the characteristics of inflammatory-cell recruitment vary in a SEB concentration-dependent manner. We also quantified the levels of IFN-γ in BAL fluid from superantigen- or PBS-challenged mice (Fig. 3D). IFN-γ levels were significantly higher in SEB 200-ng and SEB 2,000-ng groups than in either PBS or SEB 20-ng groups (P < 0.05).
To further evaluate the pathological changes in the lungs following SEB challenge, paraffin-fixed lung sections were microscopically evaluated. While PBS-treated mice showed normal lung histology (Fig. 4A), mice that received 20 ng of SEB showed marked mononuclear cell infiltration in the parenchyma as well as around the bronchi (Fig. 4C). There was goblet cell hyperplasia in the bronchi suggestive of increased mucus production. Infiltration of eosinophils around the bronchi as well as the blood vessels was also evident. Mice that received 2,000 ng of SEB showed severe damage to the alveoli as well as to the bronchi, with loss of normal lung architecture (Fig. 4F).
FIG. 4.
Histopathology induced by intranasal SEB challenge. Age-matched HLA-DR3 transgenic mice were challenged intranasally with PBS or SEB (20, 200, or 2,000 ng) on days 0, 3, and 6. After harvest of BAL fluids on day 8, lungs were collected in buffered formaldehyde and embedded in paraffin. Thin sections were stained in hematoxylin and eosin (A, C, and F), or the deparaffinized sections were stained with anti-MBP antibody (B, D, and G). (A and B) PBS-treated mice; (C to E) 20 ng SEB; (F and G) 2,000 ng of SEB. (E) Lung section from mouse that received 20 ng of SEB treated with preimmune rabbit serum followed by fluorescein isothiocyanate-conjugated secondary anti-rabbit antibody showing the specificity of the anti-MBP antibody. Magnification, ×160.
Immunostaining with anti-MBP antibody showed only occasional eosinophils in PBS-treated mice (Fig. 4B). However, mice treated with 20 ng of SEB showed marked infiltration with eosinophils, especially around the airways and blood vessels (Fig. 4D). Extracellular MBP staining was also present, indicating degranulation of eosinophils (Fig. 4D). No MBP staining was observed with mice that had received 2,000 ng of SEB.
Systemic effects of superantigen delivered by the intranasal route.
To study the systemic effect of SEB delivered intranasally, spleens and thymus glands were collected on day 8 and the distribution of CD4+ and CD8+ T cells expressing TCR Vβ6 and Vβ8 was analyzed by flow cytometry. As shown in Fig. 5A, there was a concentration-dependent increase in percentage of SEB-reactive TCR Vβ8+ CD4+ T cells (P < 0.05 for all groups except PBS versus SEB 20-ng groups, one-way ANOVA) in the spleen. No differences in SEB nonreactive TCR Vβ6+ CD4+ T cells were observed. There was a concentration-dependent reduction in TCR Vβ8+ CD8+ T cells in SEB-treated mice compared to PBS-treated mice (P < 0.05 for all groups except PBS versus SEB 20-ng groups, one-way ANOVA). Repeated intranasal delivery of even small quantities of SEB also caused significant concentration-dependent apoptosis of CD4 CD8 double-positive thymocytes (Fig. 5B). Overall, these results suggest that SEB delivered into the nasal passage causes significant systemic T-cell activation at high but not at low concentrations of SEB.
FIG. 5.
Systemic immune activation following intranasal delivery of SEB. Age-matched HLA-DR3 transgenic mice were challenged intranasally with PBS or 20, 200, or 2,000 ng of SEB on days 0, 3, and 6. Mice were sacrificed on day 8, and spleens (A) and thymuses (B) were collected for flow cytometric analysis. Each data point represents the mean ± standard deviation from 10 mice. *, P < 0.05.
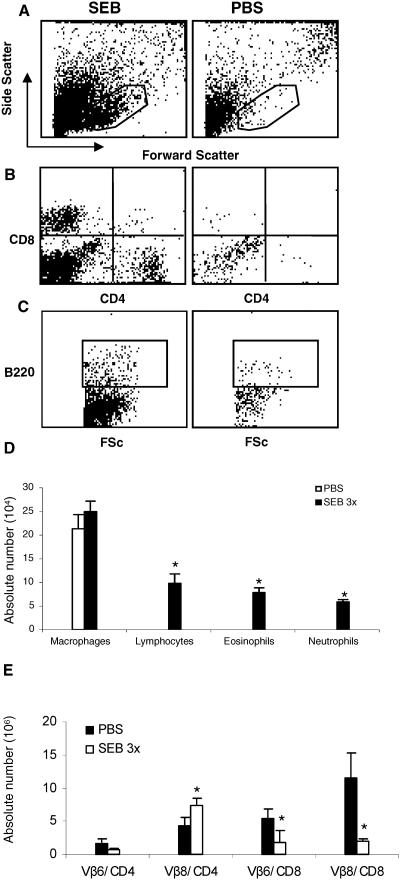
Airway inflammation in HLA-DQ8 transgenic mice following intranasal superantigen delivery.
We next confirmed the pathogenic effect of SEB on the respiratory system by using a second type of HLA class II (DQ8) transgenic mice. Since DQ molecules are less efficient in presenting SEB than are DR molecules (52), we tested only a higher concentration (1 μg) of SEB. As with HLA-DR3 transgenic mice, intranasal delivery of SEB was associated with recruitment of leukocytes into the BAL fluid (Fig. 6A to D) as well as eosinophilic airway inflammation. Flow cytometric analysis of BAL samples indicated the presence of more mononuclear cells (as represented by the gate) in mice that received SEB than in mice that received PBS. PBS-treated mice had only larger cells outside of the gate, with greater forward and side scatter, suggestive of alveolar macrophages (Fig. 6A). Further analysis of BAL fluid cells revealed the presence of CD4+ and CD8+ cells as well as B220+ cells (B cells) in mice that received SEB but not in PBS-treated mice (Fig. 6B and C). Giemsa-stained cytospin preparations revealed the presence of significantly higher numbers of lymphocytes (P < 0.05, unpaired Student's t test), eosinophils, and neutrophils in SEB-treated mice than in PBS-treated mice (Fig. 6D). As with HLA-DR3 mice, SEB-treated HLA-DQ8 mice had expansion of TCR Vβ8+ but not TCR Vβ6+ CD4+ T cells in the spleen. For unknown reasons, the CD8+ T cells were significantly reduced in SEB-treated mice (Fig. 6E).
FIG. 6.
Airway inflammation and systemic immune activation following intranasal SEB challenge in HLA-DQ8 transgenic mice. Age-matched HLA-DQ8 mice were challenged intranasally with either PBS or SEB (2,000 ng) on days 0, 3, and 6. On day 8, BAL fluid was collected for flow cytometric analysis (A to C). FSc, forward scatter. Enumeration of leukocyte subsets by use of Wright-Giemsa-stained cytospin preparations (D). Panel E represents the T-cell subsets in spleens. Each data point represents the mean ± standard error from four mice. *, P < 0.05.
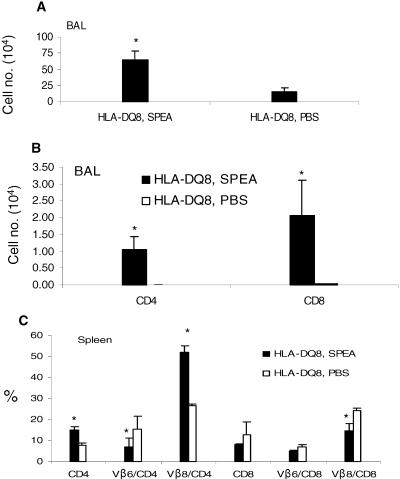
We next wanted to study if an inflammatory airway response could be induced by a different superantigen, SPEA, which is presented efficiently by HLA-DQ molecules. We tested high (1 μg/application) as well as low (20 ng/application) concentrations of SPEA. Analysis of BAL fluid and spleens indicated that SPEA induced airway inflammation as well as systemic immune activation (Fig. 7; also data not shown). Histopathological evaluation of lungs from SPEA-challenged HLA-DQ8 or C57BL/6 mice indicated that SPEA elicited very little inflammatory change in C57BL/6 mice (Fig. 8A and B). Nonetheless, SPEA at the same dose induced marked pathology in HLA-DQ8 mice (Fig. 8C and D). SPEA at 2 ng/application also caused significant inflammatory changes only in HLA-DQ8 mice (Fig. 8E and F). Increasing the concentration of SPEA to 2 μg/application resulted in higher mortality in HLA-DQ8 mice (mortality, three of five mice, or 60%).
FIG. 7.
Airway inflammation and systemic immune activation following intranasal SPEA challenge in HLA-DQ8 transgenic mice. Age-matched HLA-DQ8 mice were challenged intranasally with either PBS or SPEA (1,000 ng) on days 0, 3, and 6. On day 8, BAL fluid was collected for flow cytometric analysis (A and B). Panel C represents splenic T-cell subsets. Each data point represents the mean ± standard error from four mice. *, P < 0.05 compared to PBS group.
FIG. 8.
SPEA-induced histopathology for HLA-DQ8 mice. C57BL/6 or HLA-DQ8 transgenic mice were challenged intranasally with PBS or SPEA, and lung tissue was collected in buffered formaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. (A and B) SPEA-challenged C57BL/6 mice (1 μg/application); (C and D) SPEA-challenged HLA-DQ8 mice (1 μg/application); (E and F) SPEA-challenged HLA-DQ8 mice (2 ng/application). (A, C, and E) Lower magnification; (B, D, and F) higher magnification.
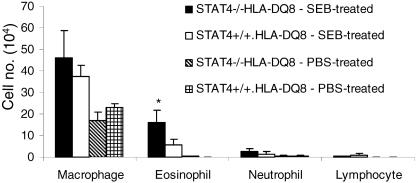
Increased eosinophil recruitment in STAT4-deficient DQ8 transgenic mice following intranasal superantigen challenge.
To study the role of interleukin-12 (IL-12) cytokine signaling in SEB-induced airway inflammation, we challenged wild-type as well as STAT4-deficient (i.e., defective IL-12 signaling) HLA-DQ8 mice with a very low concentration of SEB (20 ng/application, administered three times). There was a significant increase in eosinophil recruitment into the airways in STAT4-deficient DQ8 mice compared to levels in STAT4-sufficient HLA-DQ8 mice (P < 0.05) (Fig. 9). There was no statistically significant difference in the numbers of other cell types in BAL fluid between these two groups. Lung sections from STAT4-deficient HLA-DQ8 mice showed a heightened inflammatory response (Fig. 10B). Immunostaining with anti-MBP antibodies revealed marked infiltration of eosinophils around the airways in STAT4-deficient HLA-DQ8 transgenic mice (Fig. 10E). This was accompanied by goblet cell hyperplasia (Fig. 10B). HLA-DQ8 mice that received a similar concentration of SEB intranasally showed only milder inflammation (Fig. 10C) and minimal eosinophil infiltration (Fig. 10F). Nonetheless, the inflammatory changes in SEB-challenged DQ8 mice were significantly greater than those in PBS-challenged HLA-DQ8 mice or PBS-challenged STAT4-deficient HLA-DQ8 mice (Fig. 10A). When the SEB concentration was increased to 1 μg/application, there was a dramatic increase in the eosinophilic airway inflammation and airway changes in STAT4-deficient HLA-DQ8 mice compared to levels in STAT4-sufficient HLA-DQ8 mice (Fig. 11). Thus, disrupting STAT4 signaling changed the nature of the inflammatory cells recruited to the airways by SEB, probably by shifting the cytokine pattern from the Th1 type to the Th2 type.
FIG. 9.
Analysis of BAL fluid from STAT4-deficient and -sufficient HLA-DQ8 transgenic mice after intranasal superantigen challenge. Cytospin preparations of BAL fluid from mice challenged with SEB or PBS underwent Wright-Giemsa staining. Each bar represents the mean ± standard error from at least four different mice. *, P < 0.05 compared to SEB-treated STAT4+/+ HLA-DQ8 group.
FIG. 10.
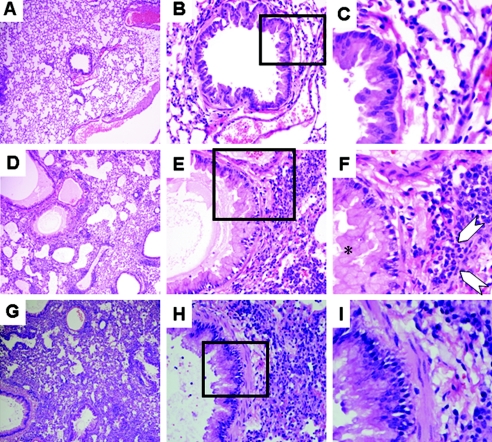
Histopathology induced by intranasal SEB challenge in STAT4-deficient mice. Age-matched STAT4-deficient or -sufficient HLA-DQ8 transgenic mice were challenged intranasally with either PBS or 20 ng of SEB on days 0, 3, and 6. Mice were sacrificed on day 8. After harvest of BAL fluids on day 8, lungs were collected in buffered formaldehyde and embedded in paraffin. Thin sections were stained in hematoxylin and eosin (A, B, and C); deparaffinized sections were stained with anti-MBP antibody (D, E, and F). (A and D) PBS-treated, STAT4-deficient DQ8 transgenic mice; (B and E) STAT4-deficient DQ8 transgenic mice treated with 20 ng SEB; (C and F) STAT4-sufficient DQ8 transgenic mice treated with 20 ng of SEB. Magnification, ×160.
FIG. 11.
Histopathology induced by intranasal SEB challenge in STAT4-deficient mice. Age-matched STAT4-deficient or STAT4-sufficient HLA-DQ8 transgenic mice were challenged with either PBS or 1 μg of SEB on days 0, 3, and 6. Mice were sacrificed on day 8, and lungs were collected in buffered formaldehyde and embedded in paraffin. Thin sections were stained in hematoxylin and eosin. (A to C) PBS-treated, STAT4-deficient DQ8 transgenic mice; (D to F) STAT4-deficient DQ8 transgenic mice treated with SEB; (G to I) STAT4-sufficient DQ8 transgenic mice treated with SEB. (A, D, and G) Lower magnification; (B, E, and H) higher magnification. (C, F, and I) Sections in the boxed areas are magnified for clarity. *, goblet cell hyperplasia; arrows, eosinophils.
DISCUSSION
Superantigenic exotoxins are important virulence factors elaborated by S. aureus and S. pyogenes (51). Bacterial superantigens have special significance in human diseases because they bind to human MHC class II molecules with high affinity (51). While the effects of superantigens ingested through the oral route (e.g., food poisoning) or absorbed through the vaginal mucosa (i.e., menstrual toxic shock syndrome) have been studied (71), the effect of superantigen exposure through the airways has not been explored. This is important because S. aureus and S. pyogenes colonize the upper airways of humans (6, 13, 97) and act as a major source of infection especially of the respiratory tract (25, 91). About 2% of community-acquired pneumonias and up to 10% of hospital-acquired pneumonias are caused by S. aureus (62). In recent years, community-acquired lung infection due to methicillin-resistant S. aureus has emerged (13, 26, 109). While it has been shown that systemically delivered bacterial superantigens can be associated with pulmonary pathology (65), it has not been known whether superantigens gaining access through the intranasal route can cause airway and/or lung inflammation.
One published study did address the role of superantigens in airway inflammation by use of a murine model (39). However, this study used a conventional murine model which has inherent limitations in terms of studying the effects of bacterial superantigens. For example, while superantigen can activate murine T cells, about a 1,000-fold-higher concentration is required to achieve activation similar to that of human T cells (22), as a result of poor binding of bacterial superantigens to murine MHC class II molecules (24, 57, 82). Therefore, the superantigen-induced airway inflammation identified in this study is likely understated. Transgenic expression of human MHC (HLA) class II molecules in mice lacking endogenous class II molecules results in a dramatic increase in their T-cell responses to bacterial superantigens (16, 20, 73-76, 82, 94, 106, 110). Therefore, we used HLA class II transgenic mice to evaluate the ability of a staphylococcal superantigen and a streptococcal superantigen administered via the intranasal route to induce inflammation of the airways and lungs.
We confirmed earlier findings that HLA class II transgenic mice mount a robust systemic in vivo immune response to bacterial superantigen compared to the conventional mouse strain BALB/c (Fig. 1). We found that while intranasal delivery of SEB induces airway inflammation in both BALB/c and HLA-DR3 transgenic mice, the magnitude of the inflammation is significantly more pronounced in the HLA-DR3 mice, even with a lower concentration of SEB (Fig. 2). Therefore, we used HLA class II transgenic mice in subsequent experiments. The nature of the airway inflammation in HLA-DR3 transgenic mice is determined by the concentration of SEB delivered intranasally. A low concentration is associated with eosinophilic airway inflammation, whereas a higher concentration is associated with a neutrophilic inflammatory response (Fig. 3 and 4). In our study, higher concentrations of SEB were associated with elevated levels of IFN-γ in the BAL fluid (Fig. 3D) and higher mortality. It has previously been established that the concentration of superantigen determines the type of the immune response elicited, namely, Th1 versus Th2 (10). Low concentrations of superantigens polarize to a Th2-type response and high concentrations to a Th1-type response (9, 61, 81, 95). Accordingly, in our study low concentrations of SEB likely induced a Th2 response, resulting in eosinophilic airway inflammation and reversible AHR, whereas high concentrations of SEB likely induced a Th1 type of response, resulting in a neutrophilic response with irreversible airway changes and higher mortality. It has been shown with other models of inflammation that a Th1-type response is associated with neutrophil recruitment (23). The extent of lung pathology induced by superantigens administered intranasally in HLA-DR3 or DQ8 mice was more severe than that reported for a conventional mouse strain (39). We have performed additional experiments using HLA-DQ6 mice and found that SEB as well as SPEA can induce airway inflammation (G. Rajagopalan et al., unpublished data). All of these observations underscore our hypothesis that intranasal exposure to bacterial superantigen can induce dose-dependent airway/lung inflammation when HLA class II molecules are expressed.
Interleukin-12 is a strong inducer of Th1-type cytokines and a potent suppressor of Th2-type cytokines. Signaling through the IL-12 receptor is mediated through STAT4 (46), and as a result STAT4-deficient mice have severe defects in IL-12 receptor-mediated signaling. STAT4-deficient mice also have defective IFN-γ production. Due to profound defects in IL-12 and IFN-γ, T cells from STAT4-deficient mice preferentially produce more Th2-type cytokines following their activation (47). In this context, a corollary between atopic individuals and STAT4-deficient mice can be made, as STAT4-deficient mice, like atopic individuals, have a tendency towards increased production of Th2-type cytokines (1, 2, 11, 42, 47, 78, 107). The increased eosinophilic airway inflammation observed with STAT4-deficient mice suggests that SEB may induce more-severe eosinophilic airway inflammation in atopic individuals than in nonatopic individuals (Fig. 9 to 11). It should be noted that in the current study, mice were exposed to SEB for a very short period of time (i.e., 1 week). This period is probably insufficient for the induction of SEB-specific immunoglobulin E (80). Since isotype switching might take longer to occur (32), the superantigen-induced eosinophilic response observed in this study is probably immunoglobulin E independent (39). Since our study was conducted with humanized mice, our results lend support to the hypothesis that superantigens could play a definite role in asthma (4), as they do in related atopic disorders such as atopic dermatitis (55) and nasal polyps (5).
Mechanistically, superantigens likely cause airway inflammation through the same mechanisms by which they cause other diseases (71). There is abundant expression of MHC class II molecules within the airways and alveoli (18). There are professional as well as nonprofessional antigen-presenting cells present in airways and lungs (18, 19, 29, 53, 99). The airways are also lined with the bronchus-associated lymphatic tissues that contain antigen-presenting cells as well as T lymphocytes (98, 101). Therefore, when the superantigens reach the airways, they may directly bind to MHC class II molecules and vigorously activate both CD4+ and CD8+ T-cell subsets bearing certain TCR Vβ families, culminating in airway inflammation. Superantigens can also directly induce mast cell degranulation and release of chemical mediators by binding to their MHC class II molecules and subsequent cross-linking (21, 72). By these mechanisms, airway exposure to superantigens may cause bronchoconstriction, mucus production, AHR, and airway inflammation.
The other interesting observation was that high concentrations of SEB delivered through the nasal mucosa caused systemic immune activation characterized by peripheral (i.e., splenic) T-cell expansion, thymic deletion, and even findings consistent with toxic shock. Absorption of SEB through mucous membranes other than that of the gut has not been demonstrated (63). Toxic shock syndrome toxin 1 is the only superantigen exotoxin known to be absorbed through nonenteric (i.e., vaginal) mucosa, with consequent menstrual toxic shock associated with the use of certain types of tampons (86). However, there are clinical observations suggesting that toxic shock syndrome may complicate respiratory tract infection (59, 93). To our understanding, ours is the first report to show that intranasal administration results in systemic immune activation and that, at higher concentrations, this mode of administration may lead to toxic shock and even death. This knowledge is important for understanding the potential effects of airway exposure to a high concentration of bacterial superantigens as a result of bioterrorism or accidental exposure (84, 92). It is well established that some bacterial superantigens interact more efficiently with certain HLA class II molecules. For example, SEB binds more efficiently to HLA-DR molecules (27, 38, 43, 87, 90) than to HLA-DQ, whereas SPEA is presented more efficiently by HLA-DQ than by HLA-DR (66, 94, 106). As a result, higher concentrations of SEB and SPEA elicited a robust immune response resulting in higher mortality in HLA-DR3 and HLA-DQ8 transgenic mice, respectively.
A recent study conducted using conventional mouse strains has proposed that mucosal exposure (i.e., through the nasal epithelium) to bacterial superantigens can induce tolerance against bacterial superantigens, which can protect against subsequent toxic shock induced by superantigens; translational use of this strategy with humans has been suggested (17). In light of our observations that intranasal exposure to superantigens can cause airway inflammation, such tolerance induction protocols should be cautiously approached.
Considering that nasopharyngeal colonization with S. aureus and S. pyogenes commonly occurs in humans and that these organisms are capable of elaborating superantigens (6, 35, 64), there is a distinct possibility that bacterial superantigens can reach the airways. Exposure to small amounts of bacterial superantigens, as might occur in the carrier state or during mild infection, could cause eosinophilic airway inflammation, especially in atopic individuals who have a tendency to mount a Th2-type immune response. Our hypothesis is strongly supported by a recent observation by Seiberling et al. (89) that at least one bacterial superantigen was detectable in nasal tissue from patients with chronic rhinosinusitis (a disease that has a strong association with nasal carriage of S. aureus [100]). They also found a strong positive correlation between eosinophil infiltration and superantigen detection. There also exists a strong positive correlation between rhinosinusitis and asthma (3). Since S. aureus and its superantigen exotoxins are associated with rhinosinusitis, superantigens may also play a role in the immunopathogenesis of asthma. Our hypothesis is further strengthened by a recent report by Okano et al., which states that intranasal administration of SEB facilitated development of allergic rhinitis in BALB/c mice when coadministered with Schistosoma mansoni egg antigen (67). Taken together, these reports provide substantial evidence that bacterial superantigens are produced in vivo. Our experimental model extends these observations and suggests that bacterial superantigens may induce airway inflammation (111). Human studies have also suggested a role for bacterial superantigens in the pathogenesis of asthma (3, 36). However, as asthma is a polygenic disease with strong environmental influence (8, 105), the low incidence of asthma compared to the carrier rate of S. aureus and S. pyogenes may be partly explained by the need for additional genetic and/or nongenetic risk factors. In conclusion, we support the hypothesis that superantigens may play a role in the etiopathogenesis of airway inflammation, including asthma. Bacterial superantigens, like SEB and SPEA, exposed through a nasal route can cause systemic immune activation and even death.
Acknowledgments
We thank Julie Hanson and her crew for excellent mouse husbandry and Michelle Smart for characterizing the transgenic mice.
This study was supported by NIH grant AI14764. G.R. is the recipient of a Juvenile Diabetes Research Foundation fellowship.
Editor: J. T. Barbieri
REFERENCES
- 1.Akira, S. 1999. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells 17:138-146. [DOI] [PubMed] [Google Scholar]
- 2.Aniansson Zdolsek, H., C. K. Janefjord, K. Falth-Magnusson, and M. C. Jenmalm. 2003. Reduced IL-2-induced IL-12 responsiveness in atopic children. Pediatr. Allergy Immunol. 14:351-357. [DOI] [PubMed] [Google Scholar]
- 3.Bachert, C., P. Gevaert, and P. van Cauwenberge. 2002. Staphylococcus aureus enterotoxins: a key in airway disease? Allergy 57:480-487. [DOI] [PubMed] [Google Scholar]
- 4.Bachert, C., P. Gevaert, and P. van Cauwenberge. 2002. Staphylococcus aureus superantigens and airway disease. Curr. Allergy Asthma Rep. 2:252-258. [DOI] [PubMed] [Google Scholar]
- 5.Bachert, C., T. van Zele, P. Gevaert, L. De Schrijver, and P. Van Cauwenberge. 2003. Superantigens and nasal polyps. Curr. Allergy Asthma Rep. 3:523-531. [DOI] [PubMed] [Google Scholar]
- 6.Becker, K., A. W. Friedrich, G. Lubritz, M. Weilert, G. Peters, and C. von Eiff. 2003. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J. Clin. Microbiol. 41:1434-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisno, A. L., M. O. Brito, and C. M. Collins. 2003. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3:191-200. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal, M. N. 2005. The role of genetics in the development of asthma and atopy. Curr. Opin. Allergy Clin. Immunol. 5:141-145. [DOI] [PubMed] [Google Scholar]
- 9.Brandt, K., J. van der Bosch, R. Fliegert, and S. Gehring. 2002. TSST-1 induces Th1 or Th2 differentiation in naive CD4+ T cells in a dose- and APC-dependent manner. Scand. J. Immunol. 56:572-579. [DOI] [PubMed] [Google Scholar]
- 10.Brogdon, J. L., D. Leitenberg, and K. Bottomly. 2002. The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naive CD4+ T cells. J. Immunol. 168:3825-3832. [DOI] [PubMed] [Google Scholar]
- 11.Camporota, L. 2001. Interleukin-12 and the development of atopy. Clin. Exp. Allergy 31:1481-1484. [DOI] [PubMed] [Google Scholar]
- 12.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, S. F. 2005. Staphylococcus aureus decolonization. Pediatr. Infect. Dis. J. 24:79-80. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, S., M. Smart, J. Hanson, and C. S. David. 2003. Characterization of HLA DR2 and DQ8 transgenic mouse with a new engineered mouse class II deletion, which lacks all endogenous class II genes. J. Autoimmun. 21:195-199. [DOI] [PubMed] [Google Scholar]
- 15.Cockerill, F. R., III, K. L. MacDonald, R. L. Thompson, F. Roberson, P. C. Kohner, J. Besser-Wiek, J. M. Manahan, J. M. Musser, P. M. Schlievert, J. Talbot, B. Frankfort, J. M. Steckelberg, W. R. Wilson, and M. T. Osterholm. 1997. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA 277:38-43. [PubMed] [Google Scholar]
- 16.Cole, B. C., A. D. Sawitzke, E. A. Ahmed, C. L. Atkin, and C. S. David. 1997. Allelic polymorphisms at the H-2A and HLA-DQ loci influence the response of murine lymphocytes to the Mycoplasma arthritidis superantigen MAM. Infect. Immun. 65:4190-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins, L. V., K. Eriksson, R. G. Ulrich, and A. Tarkowski. 2002. Mucosal tolerance to a bacterial superantigen indicates a novel pathway to prevent toxic shock. Infect. Immun. 70:2282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constant, S. L., J. L. Brogdon, D. A. Piggott, C. A. Herrick, I. Visintin, N. H. Ruddle, and K. Bottomly. 2002. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J. Clin. Investig. 110:1441-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham, A. C., J. G. Zhang, J. V. Moy, S. Ali, and J. A. Kirby. 1997. A comparison of the antigen-presenting capabilities of class II MHC-expressing human lung epithelial and endothelial cells. Immunology 91:458-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DaSilva, L., B. C. Welcher, R. G. Ulrich, M. J. Aman, C. S. David, and S. Bavari. 2002. Humanlike immune response of human leukocyte antigen-DR3 transgenic mice to staphylococcal enterotoxins: a novel model for superantigen vaccines. J. Infect. Dis. 185:1754-1760. [DOI] [PubMed] [Google Scholar]
- 21.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohlsten, M., M. Bjorklund, A. Sundstedt, G. Hedlund, D. Samson, and T. Kalland. 1993. Immunopharmacology of the superantigen staphylococcal enterotoxin A in T-cell receptor V beta 3 transgenic mice. Immunology 79:520-527. [PMC free article] [PubMed] [Google Scholar]
- 23.Fairweather, D., S. Frisancho-Kiss, S. A. Yusung, M. A. Barrett, S. E. Davis, R. A. Steele, S. J. L. Gatewood, and N. R. Rose. 2005. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-γ and macrophage and neutrophil populations in the heart. J. Immunol. 174:261-269. [DOI] [PubMed] [Google Scholar]
- 24.Fleischer, B., R. Gerardy-Schahn, B. Metzroth, S. Carrel, D. Gerlach, and W. Kohler. 1991. An evolutionary conserved mechanism of T cell activation by microbial toxins. Evidence for different affinities of T cell receptor-toxin interaction. J. Immunol. 146:11-17. [PubMed] [Google Scholar]
- 25.Foster, T. J. 2004. The Staphylococcus aureus “superbug.” J. Clin. Investig. 114:1693-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis, J. S., M. C. Doherty, U. Lopatin, C. P. Johnston, G. Sinha, T. Ross, M. Cai, N. N. Hansel, T. Perl, J. R. Ticehurst, K. Carroll, D. L. Thomas, E. Nuermberger, and J. G. Bartlett. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100-107. [DOI] [PubMed] [Google Scholar]
- 27.Fraser, J. D. 1989. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature 339:221-223. [DOI] [PubMed] [Google Scholar]
- 28.Fueyo, J. M., M. C. Mendoza, M. A. Alvarez, and M. C. Martin. 2005. Relationships between toxin gene content and genetic background in nasal carried isolates of Staphylococcus aureus from Asturias, Spain. FEMS Microbiol. Lett. 243:447-454. [DOI] [PubMed] [Google Scholar]
- 29.Glanville, A. R., H. D. Tazelaar, J. Theodore, E. Imoto, R. V. Rouse, J. C. Baldwin, and E. D. Robin. 1989. The distribution of MHC class I and II antigens on bronchial epithelium. Am. Rev. Respir. Dis. 139:330-334. [DOI] [PubMed] [Google Scholar]
- 30.Gomez, M. I., A. Lee, B. Reddy, A. Muir, G. Soong, A. Pitt, A. Cheung, and A. Prince. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10:842-848. [DOI] [PubMed] [Google Scholar]
- 31.Gorny, R. L., and J. Dutkiewicz. 2002. Bacterial and fungal aerosols in indoor environment in Central and Eastern European countries. Ann. Agric. Environ. Med. 9:17-23. [PubMed] [Google Scholar]
- 32.Gould, H. J., B. J. Sutton, A. J. Beavil, R. L. Beavil, N. McCloskey, H. A. Coker, D. Fear, and L. Smurthwaite. 2003. The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 21:579-628. [DOI] [PubMed] [Google Scholar]
- 33.Greenfield, R. A., B. R. Brown, J. B. Hutchins, J. J. Iandolo, R. Jackson, L. N. Slater, and M. S. Bronze. 2002. Microbiological, biological, and chemical weapons of warfare and terrorism. Am. J. Med. Sci. 323:326-340. [DOI] [PubMed] [Google Scholar]
- 34.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 35.Hatakka, M., K. J. Bjorkroth, K. Asplund, N. Maki-Petays, and H. J. Korkeala. 2000. Genotypes and enterotoxicity of Staphylococcus aureus isolated from the hands and nasal cavities of flight-catering employees. J. Food Prot. 63:1487-1491. [DOI] [PubMed] [Google Scholar]
- 36.Hauk, P. J., S. E. Wenzel, A. E. Trumble, S. J. Szefler, and D. Y. Leung. 1999. Increased T-cell receptor V beta8+ T cells in bronchoalveolar lavage fluid of subjects with poorly controlled asthma: a potential role for microbial superantigens. J. Allergy Clin. Immunol. 104:37-45. [DOI] [PubMed] [Google Scholar]
- 37.Heaton, T., D. Mallon, T. Venaille, and P. Holt. 2003. Staphylococcal enterotoxin induced IL-5 stimulation as a cofactor in the pathogenesis of atopic disease: the hygiene hypothesis in reverse? Allergy 58:252-256. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann, T., R. S. Accolla, and H. R. MacDonald. 1989. Different staphylococcal enterotoxins bind preferentially to distinct major histocompatibility complex class II isotypes. Eur. J. Immunol. 19:2171-2174. [DOI] [PubMed] [Google Scholar]
- 39.Herz, U., R. Rückert, K. Wollenhaupt, T. Tschernig, U. Neuhaus-Steinmetz, R. Pabst, and H. Renz. 1999. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness—a model for non-allergic asthma. Eur. J. Immunol. 29:1021-1031. [DOI] [PubMed] [Google Scholar]
- 40.Huxley, E. J., J. Viroslav, W. R. Gray, and A. K. Pierce. 1978. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am. J. Med. 64:564-568. [DOI] [PubMed] [Google Scholar]
- 41.Igwe, E. I., P. L. Shewmaker, R. R. Facklam, M. M. Farley, C. Van Beneden, and B. Beall. 2003. Identification of superantigen genes speM, ssa, and smeZ in invasive strains of beta-hemolytic group C and G streptococci recovered from humans. FEMS Microbiol. Lett. 229:259-264. [DOI] [PubMed] [Google Scholar]
- 42.Jacob, C. O., S. Zang, L. Li, V. Ciobanu, F. Quismorio, A. Mizutani, M. Satoh, and M. Koss. 2003. Pivotal role of STAT4 and STAT6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice. J. Immunol. 171:1564-1571. [DOI] [PubMed] [Google Scholar]
- 43.Jardetzky, T. S., J. H. Brown, J. C. Gorga, L. J. Stern, R. G. Urban, Y. I. Chi, C. Stauffacher, J. L. Strominger, and D. C. Wiley. 1994. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 368:711-718. [DOI] [PubMed] [Google Scholar]
- 44.Kaempfer, R., G. Arad, R. Levy, and D. Hillman. 2002. Defense against biologic warfare with superantigen toxins. Isr. Med. Assoc. J. 4:520-523. [PubMed] [Google Scholar]
- 45.Kalia, A., and D. E. Bessen. 2003. Presence of streptococcal pyrogenic exotoxin A and C genes in human isolates of group G streptococci. FEMS Microbiol. Lett. 219:291-295. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan, M. H., and M. J. Grusby. 1998. Regulation of T helper cell differentiation by STAT molecules. J. Leukoc. Biol. 64:2-5. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan, M. H., Y. L. Sun, T. Hoey, and M. J. Grusby. 1996. Impaired IL-12 responses and enhanced development of Th2 cells in STAT4-deficient mice. Nature 382:174-177. [DOI] [PubMed] [Google Scholar]
- 48.Kilburn, K. H. 2003. Summary of the 5th International Conference on Bioaerosols, Fungi, Bacteria, Mycotoxins, and Human Health. Arch. Environ. Health 58:538-542. [DOI] [PubMed] [Google Scholar]
- 49.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi, T., K. Iijima, and H. Kita. 2003. Marked airway eosinophilia prevents development of airway hyper-responsiveness during an allergic response in IL-5 transgenic mice. J. Immunol. 170:5756-5763. [DOI] [PubMed] [Google Scholar]
- 51.Kotzin, B. L., D. Y. Leung, J. Kappler, and P. Marrack. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99-166. [DOI] [PubMed] [Google Scholar]
- 52.Krakauer, T. 1999. Immune response to staphylococcal superantigens. Immunol. Res. 20:163-173. [DOI] [PubMed] [Google Scholar]
- 53.Lambrecht, B. N., H. C. Hoogsteden, and R. A. Pauwels. 2001. Dendritic cells as regulators of the immune response to inhaled allergen: recent findings in animal models of asthma. Int. Arch. Allergy Immunol. 124:432-446. [DOI] [PubMed] [Google Scholar]
- 54.Leemans, J. C., M. Heikens, K. P. van Kessel, S. Florquin, and T. van der Poll. 2003. Lipoteichoic acid and peptidoglycan from Staphylococcus aureus synergistically induce neutrophil influx into the lungs of mice. Clin. Diagn. Lab. Immunol. 10:950-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leung, D. Y., M. Boguniewicz, M. D. Howell, I. Nomura, and Q. A. Hamid. 2004. New insights into atopic dermatitis. J. Clin. Investig. 113:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li, H., A. Llera, E. L. Malchiodi, and R. A. Mariuzza. 1999. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 17:435-466. [DOI] [PubMed] [Google Scholar]
- 57.Llewelyn, M., S. Sriskandan, M. Peakman, D. R. Ambrozak, D. C. Douek, W. W. Kwok, J. Cohen, and D. M. Altmann. 2004. HLA class II polymorphisms determine responses to bacterial superantigens. J. Immunol. 172:1719-1726. [DOI] [PubMed] [Google Scholar]
- 58.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald, K. L., M. T. Osterholm, C. W. Hedberg, C. G. Schrock, G. F. Peterson, J. M. Jentzen, S. A. Leonard, and P. M. Schlievert. 1987. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA 257:1053-1058. [DOI] [PubMed] [Google Scholar]
- 60.Madsen, J. M. 2001. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin. Lab. Med. 21:593-605. [PubMed] [Google Scholar]
- 61.Marcelletti, J. F., and D. H. Katz. 1992. Antigen concentration determines helper T cell subset participation in IgE antibody responses. Cell. Immunol. 143:405-419. [DOI] [PubMed] [Google Scholar]
- 62.Marcinak, J. F., and A. L. Frank. 2003. Treatment of community-acquired methicillin-resistant Staphylococcus aureus in children. Curr. Opin. Infect. Dis. 16:265-269. [DOI] [PubMed] [Google Scholar]
- 63.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 64.Nashev, D., K. Toshkova, S. I. O. Salasia, A. A. Hassan, C. Lämmler, and M. Zschöck. 2004. Distribution of virulence genes of Staphylococcus aureus isolated from stable nasal carriers. FEMS Microbiol. Lett. 233:45-52. [DOI] [PubMed] [Google Scholar]
- 65.Neumann, B., B. Engelhardt, H. Wagner, and B. Holzmann. 1997. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J. Immunol. 158:1862-1871. [PubMed] [Google Scholar]
- 66.Norrby-Teglund, A., G. T. Nepom, and M. Kotb. 2002. Differential presentation of group A streptococcal superantigens by HLA class II DQ and DR alleles. Eur. J. Immunol. 32:2570-2577. [DOI] [PubMed] [Google Scholar]
- 67.Okano, M., H. Hattori, T. Yoshino, Y. Sugata, M. Yamamoto, T. Fujiwara, A. A. Satoskar, A. R. Satoskar, and K. Nishizaki. 2005. Nasal exposure to staphylococcal enterotoxin enhances the development of allergic rhinitis in mice. Clin. Exp. Allergy 35:506-514. [DOI] [PubMed] [Google Scholar]
- 68.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9:605-610. [DOI] [PubMed] [Google Scholar]
- 69.Petersson, K., H. Pettersson, N. J. Skartved, B. Walse, and G. Forsberg. 2003. Staphylococcal enterotoxin H induces V alpha-specific expansion of T cells. J. Immunol. 170:4148-4154. [DOI] [PubMed] [Google Scholar]
- 70.Popa, E. R., C. A. Stegeman, C. G. Kallenberg, and J. W. C. Tervaert. 2002. Staphylococcus aureus and Wegener's granulomatosis. Arthritis Res. 4:77-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Proft, T., and J. D. Fraser. 2003. Bacterial superantigens. Clin. Exp. Immunol. 133:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rago, J. V., and P. M. Schlievert. 1998. Mechanisms of pathogenesis of staphylococcal and streptococcal superantigens. Curr. Top. Microbiol. Immunol. 225:81-97. [DOI] [PubMed] [Google Scholar]
- 73.Rajagopalan, G., M. M. Sen, and C. S. David. 2004. In vitro and in vivo evaluation of staphylococcal superantigen peptide antagonists. Infect. Immun. 72:6733-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajagopalan, G., M. K. Smart, S. Cheng, C. J. Krco, K. L. Johnson, and C. S. David. 2003. Expression and function of HLA-DR3 and DQ8 in transgenic mice lacking functional H2-M. Tissue Antigens 62:149-161. [DOI] [PubMed] [Google Scholar]
- 75.Rajagopalan, G., M. K. Smart, C. J. Krco, and C. S. David. 2002. Expression and function of transgenic HLA-DQ molecules and lymphocyte development in mice lacking invariant chain. J. Immunol. 169:1774-1783. [DOI] [PubMed] [Google Scholar]
- 76.Rajagopalan, G., M. K. Smart, E. V. Marietta, and C. S. David. 2002. Staphylococcal enterotoxin B-induced activation and concomitant resistance to cell death in CD28-deficient HLA-DQ8 transgenic mice. Int. Immunol. 14:801-812. [DOI] [PubMed] [Google Scholar]
- 77.Reid, S. D., N. P. Hoe, L. M. Smoot, and J. M. Musser. 2001. Group A streptococcus: allelic variation, population genetics, and host-pathogen interactions. J. Clin. Investig. 107:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renauld, J. C. 2001. New insights into the role of cytokines in asthma. J. Clin. Pathol. 54:577-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Renz, H., and U. Herz. 2002. The bidirectional capacity of bacterial antigens to modulate allergy and asthma. Eur. Respir. J. 19:158-171. [DOI] [PubMed] [Google Scholar]
- 80.Rohde, G., P. Gevaert, G. Holtappels, I. Borg, A. Wiethege, U. Arinir, G. Schultze-Werninghaus, and C. Bachert. 2004. Increased IgE-antibodies to Staphylococcus aureus enterotoxins in patients with COPD. Respir. Med. 98:858-864. [DOI] [PubMed] [Google Scholar]
- 81.Rothoeft, T., A. Gonschorek, H. Bartz, O. Anhenn, and U. Schauer. 2003. Antigen dose, type of antigen-presenting cell and time of differentiation contribute to the T helper 1/T helper 2 polarization of naive T cells. Immunology 110:430-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy, C. J., K. L. Warfield, B. C. Welcher, R. F. Gonzales, T. Larsen, J. Hanson, C. S. David, T. Krakauer, and S. Bavari. 2005. Human leukocyte antigen-DQ8 transgenic mice: a model to examine the toxicity of aerosolized staphylococcal enterotoxin B. Infect. Immun. 73:2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudolph, M. G., and I. A. Wilson. 2002. The specificity of TCR/pMHC interaction. Curr. Opin. Immunol. 14:52-65. [DOI] [PubMed] [Google Scholar]
- 84.Rusnak, J. M., M. Kortepeter, R. Ulrich, M. Poli, and E. Boudreau. 2004. Laboratory exposures to staphylococcal enterotoxin B. Emerg. Infect. Dis. 10:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sachse, S., P. Seidel, D. Gerlach, E. Günther, J. Rödel, E. Straube, and K.-H. Schmidt. 2002. Superantigen-like gene(s) in human pathogenic Streptococcus dysgalactiae, subsp. equisimilis: genomic localisation of the gene encoding streptococcal pyrogenic exotoxin G (speGdys). FEMS Immunol. Med. Microbiol. 34:159-167. [DOI] [PubMed] [Google Scholar]
- 86.Schlievert, P. M., T. J. Tripp, and M. L. Peterson. 2004. Reemergence of staphylococcal toxic shock syndrome in Minneapolis-St. Paul, Minnesota, during the 2000-2003 surveillance period. J. Clin. Microbiol. 42:2875-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scholl, P., A. Diez, R. Karr, R. Sekaly, J. Trowsdale, and R. Geha. 1990. Effect of isotypes and allelic polymorphism on the binding of staphylococcal exotoxins to MHC class II molecules. J. Immunol. 144:226-230. [PubMed] [Google Scholar]
- 88.Schwartz, D. A., T. J. Quinn, P. S. Thorne, S. Sayeed, A. K. Yi, and A. M. Krieg. 1997. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J. Clin. Investig. 100:68-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seiberling, K. A., D. B. Conley, A. Tripathi, L. C. Grammer, L. Shuh, G. K. Haines III, R. Schleimer, and R. C. Kern. 2005. Superantigens and chronic rhinosinusitis: detection of staphylococcal exotoxins in nasal polyps. Laryngoscope 115:1580-1585. [DOI] [PubMed] [Google Scholar]
- 90.Seth, A., L. J. Stern, T. H. Ottenhoff, I. Engel, M. J. Owen, J. R. Lamb, R. D. Klausner, and D. C. Wiley. 1994. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature 369:324-327. [DOI] [PubMed] [Google Scholar]
- 91.Shah, P. M. 2001. Staphylococcus aureus in lower respiratory infections: clinical relevance of antimicrobial resistance. Semin. Respir. Infect. 16:196-202. [DOI] [PubMed] [Google Scholar]
- 92.Shailubhai, K. 2003. Bioterrorism: a new frontier for drug discovery and development. IDrugs 6:773-780. [PubMed] [Google Scholar]
- 93.Sion, M. L., A. I. Hatzitolios, E. N. Toulis, K. D. Mikoudi, and G. N. Ziakas. 2001. Toxic shock syndrome complicating influenza A infection: a two-case report with one case of bacteremia and endocarditis. Intensive Care Med. 27:443. [DOI] [PubMed] [Google Scholar]
- 94.Sriskandan, S., M. Unnikrishnan, T. Krausz, H. Dewchand, S. Van Noorden, J. Cohen, and D. M. Altmann. 2001. Enhanced susceptibility to superantigen-associated streptococcal sepsis in human leukocyte antigen-DQ transgenic mice. J. Infect. Dis. 184:166-173. [DOI] [PubMed] [Google Scholar]
- 95.Takaoka, A., Y. Tanaka, T. Tsuji, T. Jinushi, A. Hoshino, Y. Asakura, Y. Mita, K. Watanabe, S. Nakaike, Y. Togashi, T. Koda, K. Matsushima, and T. Nishimura. 2001. A critical role for mouse CXC chemokine(s) in pulmonary neutrophilia during Th type 1-dependent airway inflammation. J. Immunol. 167:2349-2353. [DOI] [PubMed] [Google Scholar]
- 96.Taneja, V., and C. S. David. 1999. HLA class II transgenic mice as models of human diseases. Immunol. Rev. 169:67-79. [DOI] [PubMed] [Google Scholar]
- 97.Toshkova, K., C. Annemuller, O. Akineden, and C. Lammler. 2001. The significance of nasal carriage of Staphylococcus aureus as risk factor for human skin infections. FEMS Microbiol. Lett. 202:17-24. [DOI] [PubMed] [Google Scholar]
- 98.Tschernig, T., and R. Pabst. 2000. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 68:1-8. [DOI] [PubMed] [Google Scholar]
- 99.Umemoto, E. Y., J. J. Brokaw, M. Dupuis, and D. M. McDonald. 2002. Rapid changes in shape and number of MHC class II expressing cells in rat airways after Mycoplasma pulmonis infection. Cell. Immunol. 220:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Zele, T., P. Gevaert, J. B. Watelet, G. Claeys, G. Holtappels, C. Claeys, P. van Cauwenberge, and C. Bachert. 2004. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J. Allergy Clin. Immunol. 114:981-983. [DOI] [PubMed] [Google Scholar]
- 101.Varoczy, L., L. Gergely, and A. Illes. 2003. Diagnostics and treatment of pulmonary BALT lymphoma: a report on four cases. Ann. Hematol. 82:363-366. [DOI] [PubMed] [Google Scholar]
- 102.Virtaneva, K., S. F. Porcella, M. R. Graham, R. M. Ireland, C. A. Johnson, S. M. Ricklefs, I. Babar, L. D. Parkins, R. A. Romero, G. J. Corn, D. J. Gardner, J. R. Bailey, M. J. Parnell, and J. M. Musser. 2005. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. USA 102:9014-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 104.von Eiff, C., A. W. Friedrich, G. Peters, and K. Becker. 2004. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 49:157-162. [DOI] [PubMed] [Google Scholar]
- 105.Weiss, S. T., and B. A. Raby. 2004. Asthma genetics 2003. Hum. Mol. Genet. 13:R83-R89. [DOI] [PubMed] [Google Scholar]
- 106.Welcher, B. C., J. H. Carra, L. DaSilva, J. Hanson, C. S. David, M. J. Aman, and S. Bavari. 2002. Lethal shock induced by streptococcal pyrogenic exotoxin A in mice transgenic for human leukocyte antigen-DQ8 and human CD4 receptors: implications for development of vaccines and therapeutics. J. Infect. Dis. 186:501-510. [DOI] [PubMed] [Google Scholar]
- 107.Wills-Karp, M. 2001. IL-12/IL-13 axis in allergic asthma. J. Allergy Clin. Immunol. 107:9-18. [DOI] [PubMed] [Google Scholar]
- 108.Wucherpfennig, K. W. 2001. Mechanisms for the induction of autoimmunity by infectious agents. J. Clin. Investig. 108:1097-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yap, F. H., C. D. Gomersall, K. S. Fung, P. L. Ho, O. M. Ho, P. K. Lam, D. T. Lam, D. J. Lyon, and G. M. Joynt. 2004. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin. Infect. Dis. 39:511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeung, R. S., J. M. Penninger, T. Kundig, W. Khoo, P. S. Ohashi, G. Kroemer, and T. W. Mak. 1996. Human CD4 and human major histocompatibility complex class II (DQ6) transgenic mice: supersensitivity to superantigen-induced septic shock. Eur. J. Immunol. 26:1074-1082. [DOI] [PubMed] [Google Scholar]
- 111.Zhang, N., P. Gevaert, T. van Zele, C. Perez-Novo, J. Patou, G. Holtappels, P. van Cauwenberge, and C. Bachert. 2005. An update on the impact of Staphylococcus aureus enterotoxins in chronic sinusitis with nasal polyposis. Rhinology 43:162-168. [PubMed] [Google Scholar]