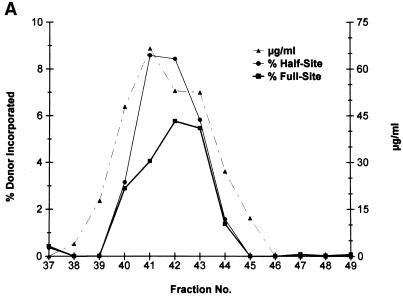
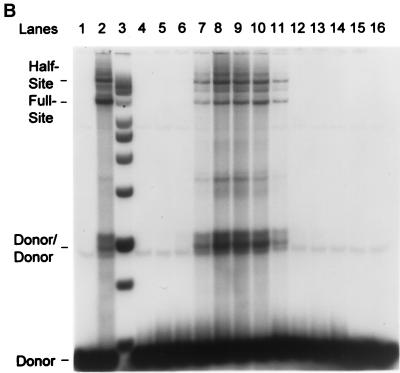
FIG. 3.
Purification of recombinant HIV-1 IN. (A) HIV-1 IN was purified by SP-Sepharose followed by heparin-Sepharose. Fractions 37 to 49 (heparin column) were analyzed for protein and strand transfer activities. The protein concentrations in fractions 40 to 44 are 48, 66, 53, 52, and 27 μg per ml, respectively (right). For fraction 41, the calculated concentration of IN is 1,030 nM. The percentages of donor incorporated into half-site and full-site strand transfer products are indicated (left; see panel B). (B) Aliquots (1 μl) of each indicated fraction shown in panel A (fractions 37 to 49 are represented in lanes 4 to 16, respectively) were preincubated with wt donor DNA (P-2) at 0°C for 12 min in 50-μl reaction volumes prior to strand transfer for 20 min at 37°C. The NaCl concentration varied from 0.63 to 0.82 M in fractions 40 to 44. Left, half-site, full-site, and donor-to-donor strand transfer products, along with the input donor. DNA products migrating slower than the half-site products contain two or more LTR donors inserted at different locations on the target DNA (40). Lanes 1 to 3, control reaction containing no IN, SIV IN at 100 nM, and molecular weight markers, respectively. The markers range in size from 0.5 to ∼10 kbp (Promega; 1-kbp DNA ladder set) (black dot, 4 kbp). (C) Aliquots (10 μl) of the indicated heparin-Sepharose fractions (lanes 1 to 9, fractions 38 to 46, respectively) were subjected to SDS-PAGE and stained with Coomassie blue.