Abstract
The relative proportions of metal residing in ore in the lithosphere, in use in products providing services, and in waste deposits measure our progress from exclusive use of virgin ore toward full dependence on sustained use of recycled metal. In the U.S. at present, the copper contents of these three repositories are roughly equivalent, but metal in service continues to increase. Providing today's developed-country level of services for copper worldwide (as well as for zinc and, perhaps, platinum) would appear to require conversion of essentially all of the ore in the lithosphere to stock-in-use plus near-complete recycling of the metals from that point forward.
Keywords: copper, material flow analysis, metal services
For at least three decades, scientists and economists have debated whether humanity is rapidly depleting the resources on which it depends (1–4). Unlike oil, which is irremediably consumed when used, metals have the potential for almost infinite recovery and reuse. Nonetheless, the rate of extraction of many geochemically scarce metals from the lithosphere has increased in excess of 3% per year through the last half century or longer and continues to do so. Because these are finite resources, it is instructive to ponder how long these extraction rates can be sustained.
As goods in use are increased and replenished, metal is transferred from the stock of ore in the lithosphere to a stock of metal-in-use providing services, and some of the metal in the original ore is transferred to wastes during mining, milling, and smelting. Over time, some of the metal-in-use recycles from old to new products, some is dissipated through corrosion and wear, and some enters waste repositories such as landfills in the end-of-life products that are not recycled. The relative sizes of the remaining stock in the lithosphere, the stock-in-use, and the stock transferred to wastes at any given time are measures of how far we have progressed toward the need for total reliance on recycling rather than on virgin ore to provide material for new products.
The demand for metal resides in the services that people receive from metal and metal-containing products, e.g., housing, transportation, and electrical power. The amount of metal in use therefore depends on the level of services and the efficiency with which metal is used in providing those services. For example, attaining a specified level of illumination in a home depends on a stock of copper in power station equipment and in transmission lines; this stock can increase if more illumination is wanted and decrease if new techniques permit the same amount of power to be generated and transmitted with less copper. Eventually the equipment and transmission lines reach the end of their service lives and are replaced. This end-of-life copper may be recycled or landfilled; if the latter, dilution with other wastes will make future recovery unlikely unless considerably improved technologies to separate metals from mixed, primarily organic, materials are implemented.
Few, if any, metals have unique properties; a substitute material can generally be found for most applications (1). Acceptance of the penalties arising from increased cost or diminished performance of a substitute material depends on the relative scarcity (or price) of the material substituted for. Thus, home illumination might be supplied through the use of aluminum rather than copper, at a cost increment arising from the lower conductivity and hence greater bulk of the aluminum that would be needed.
The continuing exploitation and the evolving use of materials require that the issue of nonrenewable resource sustainability be periodically revisited (5). Accordingly, we review here the concept of sustainability based on the stock of metal needed to provide services. Our approach allows the application of engineering data to the technical requirements of services provided in the estimation of future metal requirements and stands in contrast to economic projections such as those that estimate future requirements in terms of the flow of material per unit of the gross domestic product (6).
Calculation of Anthropogenic Metal Stocks
Archaeological, historical, and modern data show that the amounts of most metals mined and smelted before 1900 were tiny in comparison with the amounts extracted during the 20th century. Only 2.5% of the 400 Tg (1 Tg = 1 million metric tons) of copper produced during past millennia by human society was mined and smelted before 1900 (7). Much of that material, the stock, is thought to be still in use; however, the amount is not well determined.
The stock currently being used to provide services may be estimated by either a top-down or a bottom-up method. The top-down method computes the mass balance between the flow of new metal into use and the flow out of use arising from products that reach the end of their service lives. Some of the outflow of end-of-life metal is recycled into new products and so remains in use. The rest enters a steadily increasing stock placed in waste repositories and is found by integrating the balance between the discard rate and the recycling rate. Integration of the mass balance year by year determines the cumulative amount of metal stock that remains in use and the amount accumulated in wastes.
The bottom-up method begins with inventories of the different service units that contain metal, such as buildings, factories, or vehicles. The content of metal per service unit obtained from engineering data are combined with census information on the number of units in a given geographic area to determine the metal stock in use. The bottom-up method allows determination of the spatial distribution of stocks in particular localities but yields less useful data on wastes, because we lack extensive information on the metal content and extent of landfills.
Dissipation from wear and corrosion is generally small compared with the losses of metal to landfill and is unimportant in computing mass balances (8). However, dispersion of metals from wear and corrosion is an important environmental issue that can be addressed with the stock-in-use data, because dissipation rates are related to the quantities of stocks and their types of use.
Copper as an Example of Contemporary Metal Stocks
Any of the common engineering metals could be used to illustrate the link between stocks and services. We choose copper for this purpose because it is geochemically scarce (1), because its use has been studied extensively by many workers, and because the pictures it presents are representative of such metal-related resource issues as the potential for recovery from landfills and the coupling between economic growth and increased demand.
Top-Down Determinations of Copper Stock-in-Use. The amounts of copper that were extracted, entered use, and were placed in waste deposits during the 20th century in North America (Canada, the U.S., and Mexico) have been determined by Spatari et al. (9). (North America was chosen because this region was nearly self-sufficient in its copper supply during the 20th century, making allowance for imports and exports to the rest of the world, which can be difficult to determine, relatively unimportant.) Ores containing 164 Tg of copper was mined, milled, and smelted within this region through the 20th century. Historical data on process efficiencies show that ≈30 Tg of copper was lost in mine tailings and other production wastes during milling and smelting. Of the copper taken from the lithosphere, 70 Tg remain in use providing services or recycling into new products and 56 Tg has been placed in landfills. The remaining 8 Tg of copper is metal unaccounted for because of incomplete data on copper flows and trading. The stock of 70 Tg of copper in use constitutes ≈170 kg per capita, which sustains the copper services provided to people in North America.
A more detailed picture is provided by extracting the U.S. information from that for North America as a whole and determining the distribution of categories of use. From 1930 to 1939, the Bureau of Mines collected data on the destined use of new copper produced in the U.S. After an interval from 1939 through 1959 in which no one collected these data, the Copper Development Association undertook this task. Based on this information, the following four principal categories of copper use can be defined:
Building and construction comprises the copper contained in structures and is subdivided into three subcategories: interior wiring; plumbing, heating, and architectural uses; and air conditioning and commercial refrigeration.
Infrastructure, which is not subdivided and comprises copper in power-generating utilities, telecommunications, lighting, and business electronics.
Domestic and industrial equipment comprises in-plant equipment, industrial valves and fittings, nonelectrical instruments, appliances, consumer electronics, military and commercial ordnance, coinage, and off-highway vehicles. It is subdivided into domestic and industrial categories.
Transport includes two subcategories, motor vehicles (automobile, trucks and buses), and other transportation (railroad, marine, aircraft, and aerospace equipment).
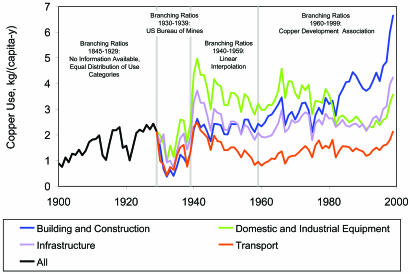
To determine flow into use throughout the 20th century, we spliced the Bureau of Mines categories into those used by the Copper Development Association and made a linear interpolation for the intervening years. Because we lack data on the distribution of copper among its different uses before 1930, we distributed the total stock in use through 1929 equally among the four categories above. Data assessment demonstrates that 92% of the total flow of copper into use in North America (166 Tg) entered products used in the U.S. The resulting flow of copper into use per capita is shown in Fig. 1. The sharp decline in the 1930s was due to economic depression; the apparent decline after 1943 arises from a period of rapid population growth. A new episode of increased per-capita flow of copper to use began in the 1990s.
Fig. 1.
Flow of copper per capita into use in the U.S. is known in aggregate to 1929 and is distributed into four use categories from 1929 onward.
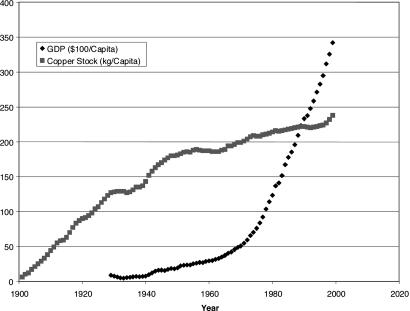
The copper stock providing services in the U.S. throughout the 20th century is computed from the difference of the flow of copper into use and out of use as determined by product lifetimes and recycling rates defined elsewhere (9, 10). Fig. 2 shows that the per-capita copper stock increased at a nearly steady rate from 1900 to 1949, except for the hiatus during the 1930s, and at a slower rate thereafter until 1995, when the rate of increase accelerated. By 1999, the stock-in-use had reached 238 kg of copper per capita.
Fig. 2.
Total per-capita copper stock in use in the U.S. through the 20th century. The GDP of the U.S. from 1929 through 1999 is also indicated.
Economists and the metals markets are, of course, interested in past and present situations primarily as guides to the future. Because economic predictions are widely available, Malenbaum (6) proposed a general relation between a nation's resource use and its gross domestic product (GDP), both expressed per capita. It was hypothesized that input would increase with GDP while a nation is building infrastructure and establishing manufactures and then decrease as the nation's economy moves more deeply into services. This hypothesis is termed the Environmental Kuznets Curve (EKC) (11). If established as a general rule, an EKC for materials could serve as the basis for estimates of future demand for metals. However, a recent study (12) finds instead, in several nations, a steady increase in the material input per capita as the GDP per capita increased, in accordance with the U.S. data in Fig. 2 but contrary to the EKC.
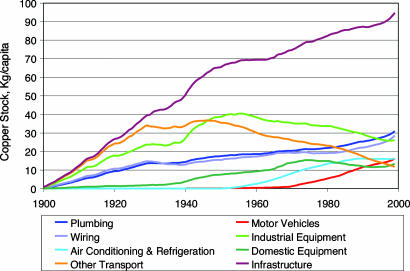
The contemporary distribution of U.S. in-use copper among the service categories is given in Table 1, and changes in categories and subcategories of the per-capita copper stock through the 20th century are shown in Fig. 3. The copper stock committed to infrastructure services (primarily power distribution and telecommunications) increased at a nearly constant annual rate throughout the 20th century. Copper stock embedded in buildings increased at a parallel but somewhat slower rate. In contrast, copper per capita devoted to equipment and to transportation reached peaks followed by declines. This decrease occurred even though people traveled more and received more delivered goods throughout the century, because the copper used in U.S. transportation systems other than motor vehicles underwent a precipitate decline after 1949 because of disinvestment in electrically powered rail and public transportation systems. The increase in the copper stock used in motor vehicles after 1970 arose from increased per-capita vehicle ownership and the higher copper content of vehicles as more electrically powered auxiliary systems were added (13). The sharp increase in copper stock in industrial equipment starting in 1940 reflects wartime expansion of manufacturing and the preeminent position of the U.S. as a manufacturing nation during the 1950s. The stock of copper invested in industrial equipment declined from 1960 onward because of deindustrialization. Copper stock in use in domestic equipment reached a plateau by 1975; its subsequent small decline may reflect increased efficiency in metal use.
Table 1. Components of copper stock-in-use in the U.S. in 1999.
| Sector or subsector | In-use stock, kg per capita |
|---|---|
| Infrastructure | 95 |
| Building and construction | 76 |
| Plumbing | 32 |
| Wiring | 28 |
| Air conditioning and refrigeration | 16 |
| Industrial and Domestic Equipment | 39 |
| Industrial | 26 |
| Domestic | 13 |
| Transportation | 28 |
| Motor vehicles | 16 |
| Railway, ships, aircraft | 12 |
| Total | 238 |
Fig. 3.
Subdivision of the copper stock per capita in the U.S. for major categories of use.
The amount of copper use per capita might indeed have been expected to decrease as engineering advances achieved dematerialization in the provision of metal services, and alternative, more abundant materials were substituted for copper in some uses, including aluminum for copper wire in high-voltage transmission lines, fiber optic for copper wire in telephone systems, and plastic for copper in many plumbing applications. However, new and expanded services that required copper, including air conditioning and enhanced motor vehicle electronics, more than offset the reduction in copper intensity achieved by increased efficiency or use of substitutes.
Bottom-Up Determinations of Copper Stock-in-Use. Several investigators, including us, have determined copper stocks for particular localities by the bottom-up method; we summarize these together with those for zinc and nickel in Table 2. Most of the bottom-up data were collected by finding the amount of metal in specific-use categories. Because each study used a different set of definitions of these categories, we cannot make direct comparisons among the data in Table 2. Some particular national differences are known to be present, however, as in the large component of the copper building and construction stock in Switzerland devoted to roofs.
Table 2. Determinations of in-use stock of various metals (kg per capita).
| Location | Cu | Zn | Ni | Source |
|---|---|---|---|---|
| Switzerland | 220 | — | — | 14 |
| United States | — | 92 | — | 15 |
| Sydney | 255 | 190 | — | 16 |
| Inner Sydney | 605 | 420 | — | 16 |
| New Haven | 144 | — | 2 | This work |
| Stockholm | 170 | 40 | 4 | 17 |
| Cape Town | 36 | 18 | — | 18 |
—, no data available.
Both the bottom-up and the top-down methods show that ≈200 kg of copper per capita are needed to supply the services enjoyed by the residents of wealthy nations, such as Switzerland, Australia, or the U.S., with current technology. [This stock-in-use determination is probably accurate to ≈15% (7).] The data also show that nations such as South Africa and China will need to increase their average urban per-capita copper stock-in-use by seven or eight times to achieve the same level of services as the developed countries if they use existing technology. Is there enough copper to meet this potential requirement?
The World Copper Resource and Future Demand
Prospecting, mining experience, and geological data define the stock of metal in the lithosphere at accessible depths. These data are conventionally presented as the reserves and reserve base of a metal. The reserve base is the stock of metal in the lithosphere that meets some minimum physical and chemical criteria related to current mining and milling practice; it includes components that are subeconomic (19). The economically viable fraction of the reserve base constitutes the reserves, defined in terms of current prices and costs of production; this is the material presently being mined or likely to be. Different choices of criteria lead to divergent resource estimates by different investigators. For example, the U.S. Geological Survey places the world copper reserve base at 950 Tg (20), whereas Grassmann and Meyer (21) report 548 Tg.
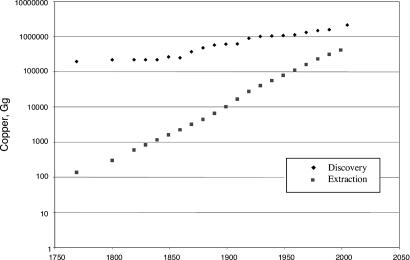
The reserve base fails as an indicator of the long-term availability of a metal resource because its definition is tied to current technology and economics. Improved techniques in mining and milling have allowed both the reserve base and reserves of the nonferrous metals to expand year by year, often at about the same rate as the rate of extraction. The increases in reserve base include new discoveries and reclassification of some of the metal stock in the lithosphere that meets new technological or economic criteria. We can test the relative contributions of new discoveries and reclassification in the reserve base of copper with historical data on the rate of discovery of new copper resources. Since 1925, the rate of increase of discovered new copper stock in the lithosphere has been 0.63% per year, significantly less than the rate of increase of the reserve base (3.9% per year) and of the stock in use (3.3% per year) (Fig. 2). Thus, although the increase in the reserve base has kept pace with the rate of extraction, only about a sixth of this increase has resulted from the discovery of new sources of copper.
A year-by-year comparison of the stock of copper in discovered resources and the amount of metal removed from this stock can indicate future scarcity if the discovery rate is less than the extraction rate. The cumulative discovery of copper resources in the lithosphere from 1750 to 1999 has been estimated with the aid of a compilation of all porphyry copper mines worldwide (22, 23). (Porphyry deposits, consisting of large crystals in a fine-grained igneous matrix, are the principal sources of the world's copper.) The result as of 1999 is 1,393 Tg of copper. Nonporphyry deposits make an additional contribution to the overall resource estimated to be ≈220 Tg. To allow for the undated and the nonporphyry copper resources, the copper in these deposits was distributed uniformly among dated deposits to construct Fig. 4, which indicates that the total copper resource is ≈1,600 Tg. Because of the nature of the available data sources, it is unlikely that the accuracy of this estimate can be much improved in the near future.
Fig. 4.
Cumulative discovery of copper in ore and the cumulative extraction of copper worldwide in the 18th–20th centuries.
A first-order application of the stocks-for-services hypothesis can be used to estimate future metal demand for copper by supposing that, as less-developed nations approach the level of services enjoyed by the developed nations, they will eventually require a similar level of metal stock per capita. The Intergovernmental Panel on Climate Change Special Report on Emission Scenarios (24) predicts a world population of 8.7 × 109 by ≈2050 and 10 × 109 to 15 × 109 by 2100. To provide each of 10 × 109 persons with a stock of 170 kg of copper, the per-capita average for North America (9), would require 1,700 Tg of copper. To attain this level of stock-in-use, more than the total world copper resource of ≈1,600 Tg would have to be placed in a sustainable cycle. Even more copper would be required to attain the per-capita level of stock for the U.S. and other wealthy Western nations. Additionally, our data show that the copper stock per capita in use in the U.S. continued to increase throughout the 20th century. No end to expansion of demand for copper services is yet in sight.
The copper stock per capita needed to provide services can potentially be reduced by more efficient metal use through better product design. The amount of new copper required to produce these services can also be reduced by designing products for more efficient recycling. A third alternative is substitution of more abundant materials for copper. This last approach is often technically feasible but not always cost-effective at current metal prices. Because the real price of copper has remained low for many decades (1), there has been little economic incentive to use substitute materials for services now supplied by copper.
Other Geochemically Scarce Metals
A recent U.S. Geological Survey compilation of historical data on metals production since 1900 (25) supplements Schmitz's (23) compilation and enables us to compute the global extraction rates of geochemically scarce metals from the lithosphere, as shown in Table 3. For the time intervals listed, the extracted stock, S, can be represented by S = SoekT, with an annual growth rate, k, and doubling time, td, for the interval. It is of interest here that the rates of growth of the stocks of extracted zinc (k = 4.2% per year, 1849–1909), nickel (k = 5.9% per year, 1939–1979) and tin (steadily decreasing from 1900 to 1959) were higher in the years before the rates shown in Table 3 were attained. It appears that each of these metals found new applications in the decades after it first became available at low cost and that, as these uses matured, extraction settled into a steady rate of increased growth. For example, the rate of increase of the extracted platinum stock has been steady at k = 4.9% per year except between 1959 and 1979, when k = 7.4% per year. This increment in the platinum stock in use arose largely from the introduction of catalytic converters on motor vehicles.
Table 3. Growth of extraction rates of geochemically scarce metals.
| Metal | k, % per year | td, years |
|---|---|---|
| Copper (1910-2002) | 3.3 | 21 |
| Zinc (1910-2002) | 3.2 | 22 |
| Nickel (1979-2002) | 3.8 | 18 |
| Tin (1959-2002) | 1.8 | 39 |
| Platinum (1979-2002) | 4.9 | 14 |
The calculations were performed for the indicated ranges of dates (for which data were available).
Zinc: A Story of Dissipative Uses. As with copper, the use of zinc increased rapidly over the last century and a half. Jolly (15) estimated that, of 73 Tg of zinc placed in service in the U.S. between 1850 and 1990, 23 Tg remains in use, only 4 Tg were recycled, and 46 Tg (63%) were lost in waste repositories or were dissipated. This situation occurs because, unlike copper and most other metals, the important uses of zinc are inherently dissipative, as in galvanizing (zinc plating of steel) and brass in brake linings. We estimate the rate of dissipation from this zinc stock by using data for Stockholm, which has a zinc stock of 28 Gg (17). Palm and Östlund (26) report emissions of 30 Mg (1 Mg = 1 metric ton) of zinc per year from Stockholm, a rate of 1.1 × 10–3 per year from stock. If this rate applies worldwide, the emission rate from zinc-in-service into the environment is 117 Gg per y, and the stock of zinc remaining in use from the cumulative world production of zinc in 2002 (346 Tg) is 109 Tg.
The global zinc resource is estimated with less certainty than is the case for copper, but the U.S. Geological Survey (20) gives 1,800 Tg of zinc as a working figure. The most recent estimate of in-use zinc stock in a wealthy country is ≈200 kg per capita (16). Were all of the world's peoples to employ zinc at the current wealthy country rate, and with contemporary technology, 2,000 Tg of zinc would be needed. Maintaining an in-use resource of this magnitude by cycling of the metal through different anthropogenic uses would be difficult so long as dissipative uses remain important in the ways in which zinc is used.
Platinum: Catalysts in Automobiles and Fuel Cells. The stock of platinum in the lithosphere can be estimated with more confidence than that of other metals because its occurrence is restricted to just two geological settings. Platinum's particular geochemical properties concentrate it above its average geochemical abundance only in chromite horizons and sulfide-rich layered intrusions that contain nickel, chromium, and copper in mafic and ultramafic rocks. Platinum is also recovered from placers formed by the weathering of these rocks (27). Because of the distinctive characteristics of platinum deposits, geologists consider it unlikely that significant new platinum resources will be found.
In a review of world platinum deposits Råde (28) found 66.5 Gg of identified platinum resources. Our independent assessment has yielded 97.1 Gg of platinum-group metals as the total resource of which 67.3 Gg containing 37 Gg of platinum remains unmined. Råde reported 90% recovery in mining and 88% efficiency in milling and smelting, which indicates that a total of 29 Gg of platinum-group metals is available for future use.
Platinum is prized as a jewelry metal, but it is also a superb catalyst. It is widely used in automobile exhaust systems (1–5 g per vehicle) and in a variety of industrial applications. Suppose that the 500 million vehicles estimated to be in use worldwide in 2000 were converted to fuel cell operation operating on pure hydrogen (i.e., no reforming of fuel needed), that the platinum requirement was 0.4 g/kW, that the average vehicle power was 75 kW, that the fuel cell life was 10 years with a 90% recycling rate, and that recycling achieved 50% recovery of the platinum content. The platinum stock-in-use for these vehicles would be 15 Gg. Maintaining this stock would require a flow of new metal into use of ≈1 Gg per year. If all of the remaining lithospheric stock of platinum were devoted to operating a fleet of 500 million vehicles with fuel cells, the platinum resource in the lithosphere would sustain this fleet for ≈15 years. There would be competition for this platinum for use in jewelry, stationary power fuel cells, industrial catalysts, and catalytic converters for motor vehicles still using petroleum fuel.
Tin: The Transition to Lead-Free Solder. Tin has long been used as an anticorrosion coating on steel, forming the “tin can.” That use is still tin's largest, but the lighter aluminum can is displacing the tin can for many uses. Tin is also used for wood preservation, antifouling paints, as a component of bronze, and as an additive to minimize the discoloration and embrittlement of a variety of plastics (29). Tin's other major use, rapidly approaching dominance, is as the principal component of electronic solders, which have traditionally had a composition of 60% tin/40% lead ratio by weight. The electronics industry is now moving toward very high-concentration tin solder formulations that avoid the use of toxic lead but must meet a complex set of physical requirements, including a low melting point, good “wetting” properties, freedom from “whisker” growth, and stability in service (30, 31). An alloy that meets these conditions is a tin–silver–copper eutectic consisting of ≈96% (by weight) tin, 3% silver, and 1% copper.
The estimated consumption of tin in electronic solder in the U.S. is 9.2 Gg per year (20). If the ratio of tin use in the U.S. to that worldwide applies to electronic solder, the world use of tin for this purpose would be 41 Gg per year. Conversion to lead-free solder based on tin with small alloy additions would increase the use of tin to ≈68 Gg per year. However, world tin reserves are 7 Tg and the reserve base is 10 Tg. We lack data on tin's in-use stock or on the total resource (32), but it appears unlikely that conversion to lead-free solder would encounter any limitations in the tin supply.
Silver: Digital Photography Versus Electronics. Silver has been prized for millennia as a semiprecious metal, especially suitable for ornamental purposes. Even today, about a quarter of its use is for jewelry and silverware (33). Another quarter is used for a variety of industrial applications, and a third quarter for photography (silver halide crystals being the classic initiator of the photographic image). Silver is used as a conductor in electronics (10–15% of its total use), and in small amounts for a variety of specialty solders.
With the rise of digital photography, one of silver's classical uses is in decline. The onset of lead-free solder, however, has opened up a new use for this historic material. Global use of 95% tin/3% silver/1% copper solder has the potential to increase the use of silver in solder by perhaps seven or eight times today's levels. Thus, one of silver's major uses is decreasing, another increasing, and its overall rate of use in the next few decades somewhat uncertain.
No estimates of the silver resource exist, but because silver often occurs geologically with zinc and copper, a rough estimate of the magnitude of the global resource is provided by multiplying the zinc resource by the ratio of the silver crustal abundance to the average of the zinc and copper crustal abundances (34). The result is ≈4 Tg of silver or ≈400 g of silver per capita for the anticipated global population in 2050. No in-use stock estimates for silver have been made, but a recent determination of silver cycles in many countries (35) indicates annual additions to stock in wealthy countries of 5–7 g per capita per yr. If this rate were applied globally, the silver resource would satisfy needs for more than half a century, so silver appears not to have an imminent resource constraint.
Nickel: Envisioning the Outlook for Stainless Steel. Some 70% of nickel is used as a constituent of stainless steel, where its various applications include tableware, appliances, and a wide variety of industrial uses. The remainder is roughly equally divided between high-temperature, corrosion-resistant alloys with copper and other metals and as a plating for steel (29).
Stainless steel use is clearly related to wealth, being widely used on “upscale” buildings, appliances, high-tech industries, and the like. Nickel's estimated resource is 130 Tg (32), or ≈13 kg of nickel per capita for the expected year 2050 population. We recently estimated the in-use nickel stock in New Haven, CT, to be ≈2 kg per capita, so we see no looming nickel supply constraints over the long term.
Conclusions
The fraction of the stock of recoverable resources in the lithosphere already placed in use or in wastes from which it will probably never be recovered is currently ≈26% for copper and 19% for zinc. We lack data, but suggest that similar proportions apply for the other industrially important, geochemically scarce metals. Because the remaining stocks of ore are large compared with current needs, prices of these metals do not yet reflect scarcity value. Additionally, improved extraction techniques have kept the average real prices of these metals nearly steady for over 50 years (1). There is no immediate concern about the capacity of mineral resources to supply requirements for the geochemically scarce metals. Limitations would arise only from restrictions on international trade or legislative restrictions related to the environmental consequences of mining, milling, and smelting lower-grade ores (1–5). Nonetheless, over time the widespread adoption of certain new technologies can be expected to encounter natural limitations in cases for which a particular material provides a unique service. We identify platinum as the most likely metal to face this limitation because of its unique catalytic properties and its desirability for such applications as alloys for high-temperature service.
Data on the stock of copper used in the U.S. over the past century cast doubt on the idea that demand for metals eventually decreases as incomes rise. Although the nation's GDP has increased much faster than the copper stock-in-use, the rate of increase of the per-capita copper stock remains undiminished. We find that the per-capita copper committed to some services has decreased in the 20th century but that this decrease is overbalanced by the provision of new services. The demand for new services is deeply embedded in a western popular and political culture that sees growth and development as absolutes, quickly converting services originating as luxuries or entertainments for the wealthy into necessities for everyone. Scenarios depicting future use of copper resources anticipate worldwide spread of the metal services enjoyed by the postindustrial nations. These scenarios need to explicitly address the cultural factors that continue to increase the per-capita use of copper in wealthy societies and the use of alternative materials to provide copper services.
Concern about the extent of mineral resources arises when the stock of metal needed to provide the services enjoyed by the highly developed nations is compared with that needed to provide comparable services with existing technology to a large part of the world's population. Our stock data demonstrate that current technologies would require the entire copper and zinc ore resource in the lithosphere and perhaps that of platinum as well. Even a lower level of services could not be sustained worldwide because a continuing supply of new metal is needed to make up for inevitable losses in the recycling of the metal stock-in-use. Substitution has the potential to ameliorate this situation, but one should not automatically assume that technology will produce a satisfactory substitute for every service at an affordable price and precisely when needed.
The topic of resource constraints inevitably recalls the classic bet between Julian Simon and Paul Ehrlich in 1980, in which Ehrlich bet that the prices of five metals would increase by 1990 (36). Instead, the grouped prices fell, and Ehrlich paid Simon $576.07 to settle the wager. Unlike Ehrlich, we do not imply that metal price is a satisfactory measure of the remaining amount of a resource. Rather, we merely point out the present state of affairs: that anthropogenic and lithospheric stocks of at least some metals are becoming equivalent in magnitude, that worldwide demand continues to increase, and that the virgin stocks of several metals appear inadequate to sustain the modern “developed world” quality of life for all Earth's peoples under contemporary technology. These facts compel us to ask two key questions: Do we really envision a developed world quality of life for all of the people of the planet? and If so, are we willing to encourage the transformational technologies that will be required to make that vision a reality?
Notwithstanding the answers to the key questions posed above, it is clear that, as the proportion of the stock of ore remaining in the lithosphere diminishes relative to the stock-in-use and the stock dissipated, scarcity value will indeed eventually raise the real prices of the geochemically scarce metals and will stimulate intensive recycling well above today's levels (37). We anticipate that price increases are unlikely to trigger a lower rate of increase in metal services or sudden economic disruption. More likely, we will see a new engineering emphasis on using these metals more efficiently and increased use of abundant alternative materials, principally iron and its alloys, aluminum, and magnesium. We anticipate a gradual transition to reliance on these alternative materials, with the use of the scarce metals increasingly restricted to those services most difficult to obtain by material substitution.
Acknowledgments
We thank R. Lifset, D. Müller, B. Reck, and B. Skinner for helpful discussions; K. Drakonakis, J. Rauch, and K. Rostkowski for assistance in field research; and J. Cao for assistance in graphics preparation. Portions of this research were supported by U.S. National Science Foundation Grant BES-0329470 and by the Nickel Institute.
Author contributions: R.B.G. and T.E.G. designed research; R.B.G. and M.B. performed research; R.B.G. and M.B. analyzed data; and R.B.G. and T.E.G. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: GDP, gross domestic product.
References
- 1.Tilton, J. E. (2003) On Borrowed Time? Assessing the Threat of Mineral Depletion (Resources for the Future, Washington, DC).
- 2.Hodges, C. A. (1995) Science 268, 1305–1312. [DOI] [PubMed] [Google Scholar]
- 3.Grant, L. (2005) Science 309, 53–54. [Google Scholar]
- 4.Ehrenfeld, D. (2005) Science 309, 54–56. [DOI] [PubMed] [Google Scholar]
- 5.Simpson, R. D., Toman, M. A. & Ayres, R. U., eds. (2005) Scarcity and Growth Revisited: Natural Resources and the Environment in the New Millenium (Resources for the Future, Washington, DC).
- 6.Malenbaum, W. (1978) World Demand for Raw Materials in 1985 and 2000 (McGraw–Hill, New York).
- 7.Lifset, R. J., Gordon, R. B., Graedel, T. E., Spatari, S. & Bertram, M. (2002) J. Mineral. Metals Mater. Soc. 54, 21–26. [Google Scholar]
- 8.Ayres, R. U., Ayres, L. W. & Råde, I. (2002) The Life Cycle of Copper, Its Co-Products and By-Products (World Bus. Council Sustain. Dev., Geneva).
- 9.Spatari, S., Bertram, M., Gordon, R. B., Henderson, K. & Graedel, T. E. (2005) Ecol. Econ. 54, 37–51. [Google Scholar]
- 10.Zeltner, C., Bader, H.-P., Scheidegger, R. & Baccini, P. (1999) Reg. Environ. Change 1, 31–46. [Google Scholar]
- 11.Cleveland, C. J. & Ruth, M. (1999) J. Ind. Ecol. 2 (3), 15–50. [Google Scholar]
- 12.Bringezu, S., Schütz, H., Steger, S. & Baudisch, J. (2004) Ecol. Econ. 51, 97–124. [Google Scholar]
- 13.Pinhao, C. M. (1996) Energia e noves materiasis: o caso das firbra oticas (Mestre Prog. Planejamento Energet., Rio de Janiero, Brazil).
- 14.Wittmer, D., Lichtensteiger, T. & Baccini, P. (2003) in Copper 2003 (Can. Inst. of Mining, Metal., and Petrol., Montreal), pp. 85–101.
- 15.Jolly, J. H. (1002) Materials Flows of Zinc in the United States 1850–1900, Open File Report 72-92 (U.S. Bureau of Mines, Washington, DC).
- 16.van Beers, D. & Graedel, T. E. (2005) J. Cleaner Prod., in press.
- 17.Sörme, L., Bergbäck, B. & Lohm, U. (2001) Water, Air, Soil Pollut. 1, 197–211. [Google Scholar]
- 18.van Beers, D. & Graedel, T. E. (2003) S. Afr. J. Sci. 99, 61–69. [Google Scholar]
- 19.U.S. Geological Survey. (2002) 1998 Assessment of Undiscovered Deposits of Gold, Silver, Copper, Lead, and Zinc in the United States, Circular 1178 (U.S. Geol. Survey, Washington, DC).
- 20.U.S. Geological Survey. (2002) Mineral Yearbook (U.S. Geol. Survey, Washington, DC).
- 21.Meyer, F. M. & Grassmann, J. (2003) Erzmetall 56, 349–355. [Google Scholar]
- 22.Singer, D. A. (2002) Porphyry Copper Deposits of the World, Open File Report 02-268 (U.S. Geol. Survey, Washington, DC).
- 23.Schmitz, C. J. (1979) World Non-ferrous Metal Production and Prices, 1700–1976 (Cass, London).
- 24.Intergovernmental Panel on Climate Change (2000) Emission Scenarios (Cambridge Univ. Press, Cambridge, U.K.).
- 25.Kelly, T., Buckingham, D., DiFrancesco, C., Porter, K., Goonan, T., Sznopek, J., Berry, C. & Crane, M. (2001) Historical Statistics for Mineral and Material Commodities in the U.S., Open File Report 2001-006 (U.S. Geol. Survey, Washington, DC).
- 26.Palm, V. &Östlund, C. (1996) Sci. Total Env. 192, 95–109. [Google Scholar]
- 27.Craig, J. R., Vaughan, D. J. & Skinner, B. J. (2001) Resources of the Earth (Prentice–Hall, Englewood Cliffs, NJ).
- 28.Råde, I. (2001) Dissertation (Chalmers Univ. of Tech., Göteborg, Sweden).
- 29.Kesler, S. (1994) Mineral Resources, Economics, and the Environment (Macmillan, New York).
- 30.Subramanian, K. N. & Lee, J. G. (2003) J. Mineral. Metals Mater. Soc. 55 (5), 26–32. [Google Scholar]
- 31.Schoenung, J. M., Ogunseitan, O. A., Saphores, J.-D. M. & Shapiro, A. A. (2004) J. Ind. Ecol. 8, 59–85. [Google Scholar]
- 32.U.S. Geological Survey. (1996) Commodity Summaries (U.S. Geol. Survey, Washington, DC).
- 33.Silver Institute and Gold Field Mineral Services (2002) World Silver Survey (Silver Inst. Gold Field Mineral Services, Washington, DC).
- 34.McLennan, S. M. (2001) Geochem. Geophys. Geosys. 2, Art. no. 2000GC000109.
- 35.Johnson, J., Jirikowic, J., Bertram. M., van Beers, D., Gordon, R. B., Henderson, K., Klee, R. J., Lanzano, T., Lifset, R., Oetjen. L., et al. (2005) Environ. Sci. Technol. 39, 4655–4665. [DOI] [PubMed] [Google Scholar]
- 36.Botkin, D. B., Keller, E. A. (2005) Environmental Science: Earth as a Living Planet (Wiley–Interscience, New York), 5th ed.
- 37.Henstock, M. E. (1996) The Recycling of Non-Ferrous Metals (Int. Council Metals Environ., Ottawa).