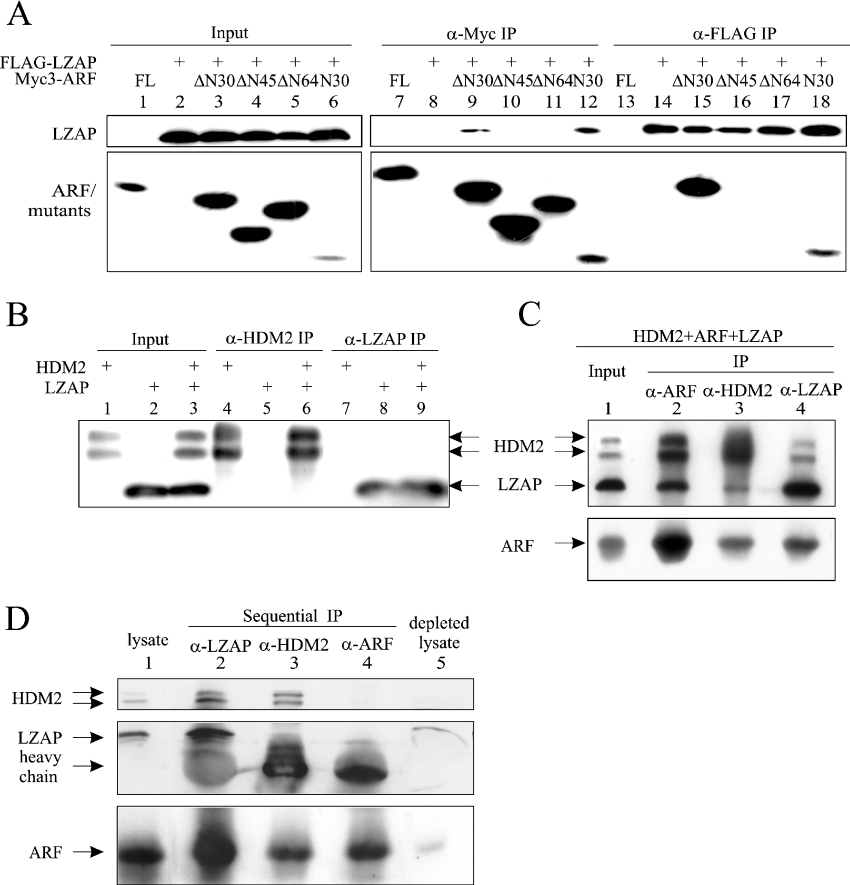
Figure 3. LZAP binds to the N-terminal region of ARF, and forms a ternary complex with ARF and HDM2.
(A) U2OS cells were transfected with pcDNA3-FLAG-LZAP and pcDNA3-Myc3-ARF (full-length or deletion mutants, see text for details) as indicated. Protein complexes were immunoprecipitated (IP) with anti-FLAG (α-FLAG) (M2) or anti-Myc (α-Myc) (9E10) antibodies, and proteins were detected by blotting with HRP-conjugated M2 or 9E10 antibodies. The Myc3-tagged ΔN64–ARF revealed an unusually slower mobility than the Myc3-tagged ΔN45–ARF, with relevant plasmids verified by direct sequencing. (B) U2OS cells were transfected with pcDNA3-LZAP and/or pCMV-HDM2. Immunoprecipitates (IP) were prepared by antibodies recognizing HDM2 (α-HDM2) (SMP14) or LZAP (α-LZAP) (3486) before SDS/15% PAGE and immunoblotting with the corresponding antibodies. (C) U2OS cells were transfected as indicated and immunoprecipitates (IP) were prepared by antibodies specifically recognizing HDM2 (α-HDM2), LZAP (α-LZAP) or ARF (α-ARF), separated by SDS/15% PAGE and immunoblotted with corresponding antibodies. (D) LZAP, HDM2 and ARF were sequentially depleted from 250 μg of cell lysate as described in the Materials and methods section. Immunoprecipitates (IP), as well as the original and depleted lysates, were subjected to SDS/15% PAGE and immunoblotting.