Figure 5. Enzymatic characterization of mouse RP2p.
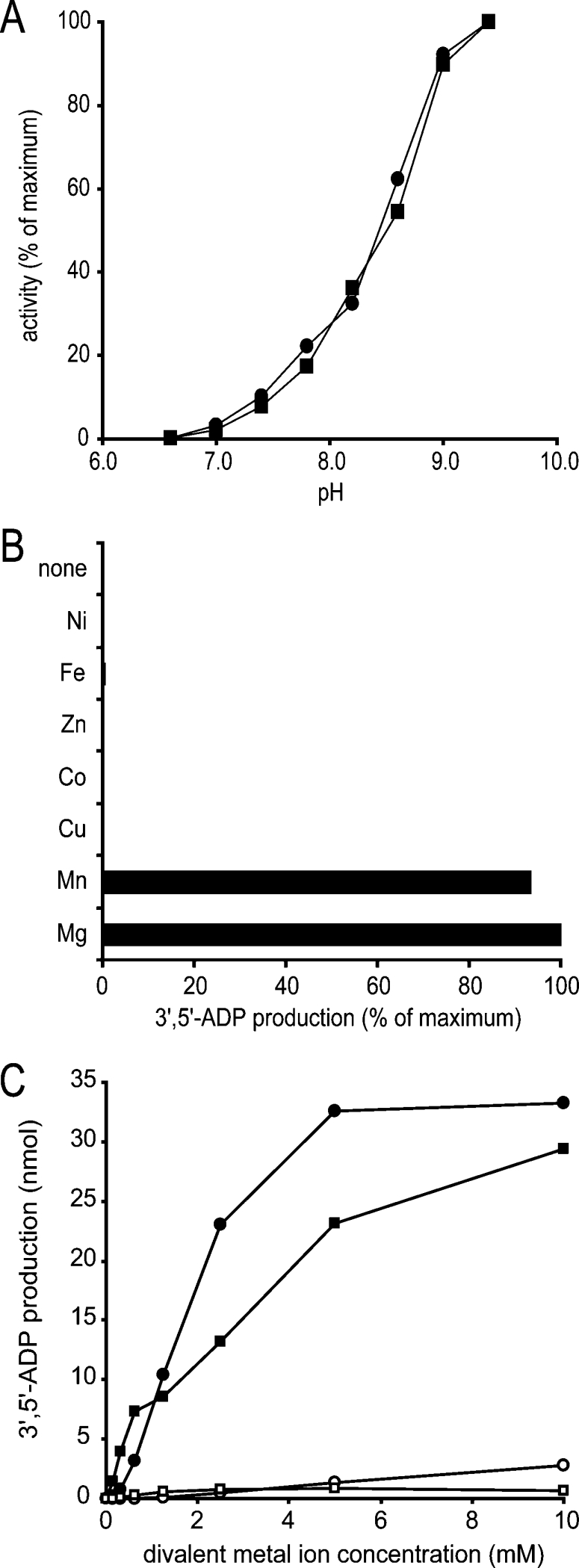
(A) The pH-dependency of CoA diphosphatase activity of purified mouse kidney peroxisomes (■, 10 μg protein) and recombinant mouse RP2p (●, 2.8 μg of protein) was determined under the assay conditions as described in the Experimental section, except that Tris/HCl buffer was replaced with glycine/Hepes buffer (100 mM each) at the indicated pH. (B) Requirement of bivalent metal ions for CoA diphosphatase activity. Purified recombinant mouse RP2p (2.8 μg of protein) was incubated under the standard assay conditions as described in the Experimental section in the presence or absence of several bivalent metal ions as indicated on the left at a final concentration of 5 mM. (C) Mg2+ and Mn2+ ion dependency of CoA diphosphatase activity. CoA diphosphatase activity was measured under the conditions described above in the presence of Mg2+ (●,○) or Mn2+ (■,□) at the indicated concentrations using a Tris/HCl buffer at pH 9.0 (■,●) or 7.4 (□,○).
