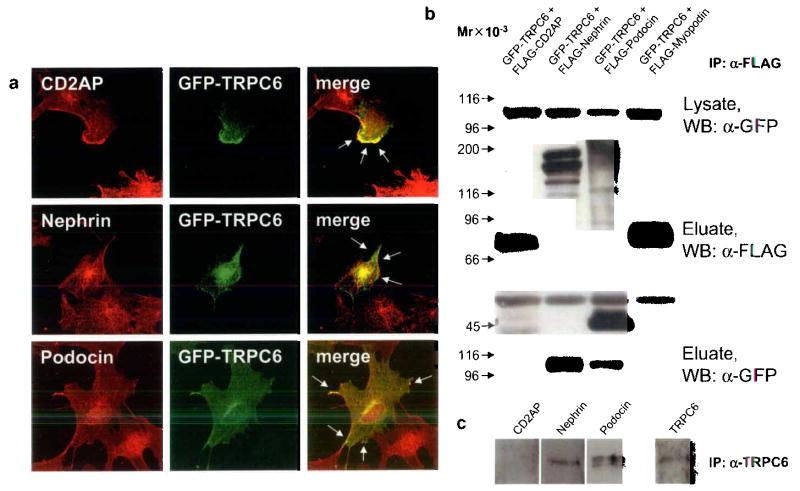
FIGURE 2.
TRPC6 co-localizes and directly interacts with slit diaphragm proteins. (a) GFP-TRPC6 co-localizes with CD2AP, nephrin and podocin at the cell membrane of cultured podocytes as shown by confocal microscopy (arrows). (b) GFPTRPC6 associates with FLAG-tagged nephrin and podocin but not with CD2AP in co-transfected HEK293 cells. GFP-tagged TRPC6 was detected in total cell lysate by immunoblotting using an anti-GFP antibody (upper panel). FLAG-tagged fusion proteins were immunoprecipitated, eluted and visualized with an anti-FLAG antibody (middle panel). Co-immunoprecipitated GFP-TRPC6 was detected in eluate fractions (lower panel). FLAG-myopodin served as a negative control for TRPC6-binding. (c) Endogenous co-immunoprecipitation of TRPC6 with slit diaphragm proteins from cultured podocytes. TRPC6 interacts with nephrin and podocin but not with CD2AP. Mr, relative molecular mass; WB, primary antibody used for Western Blotting.