Fig 1.
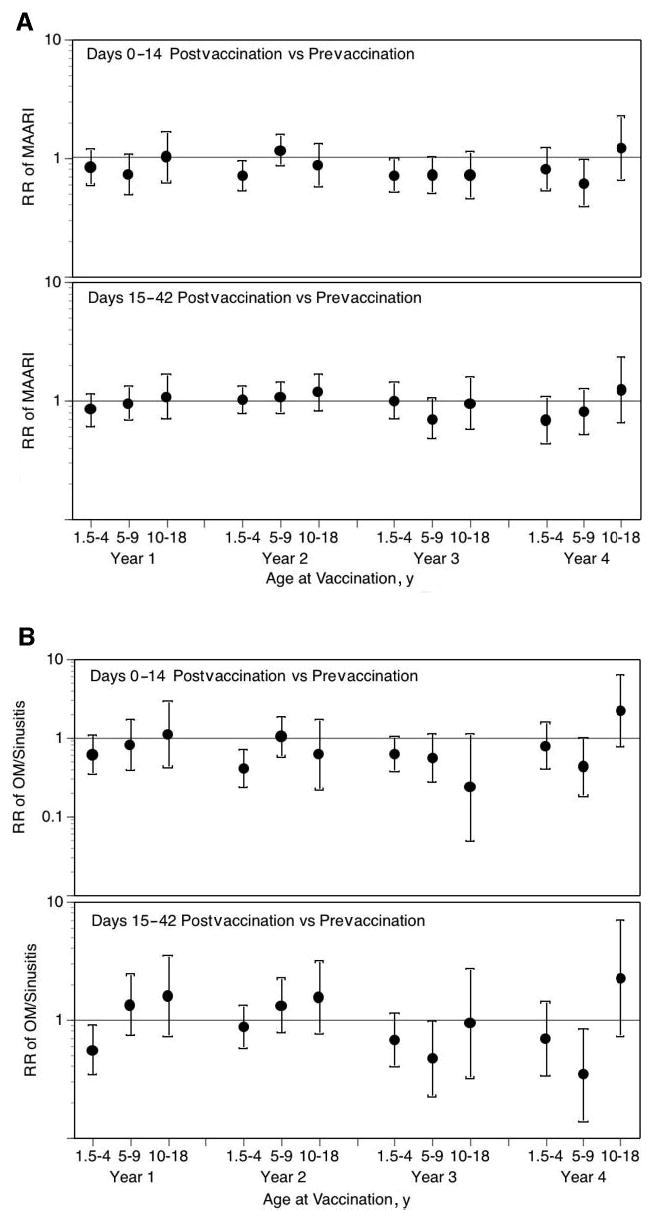
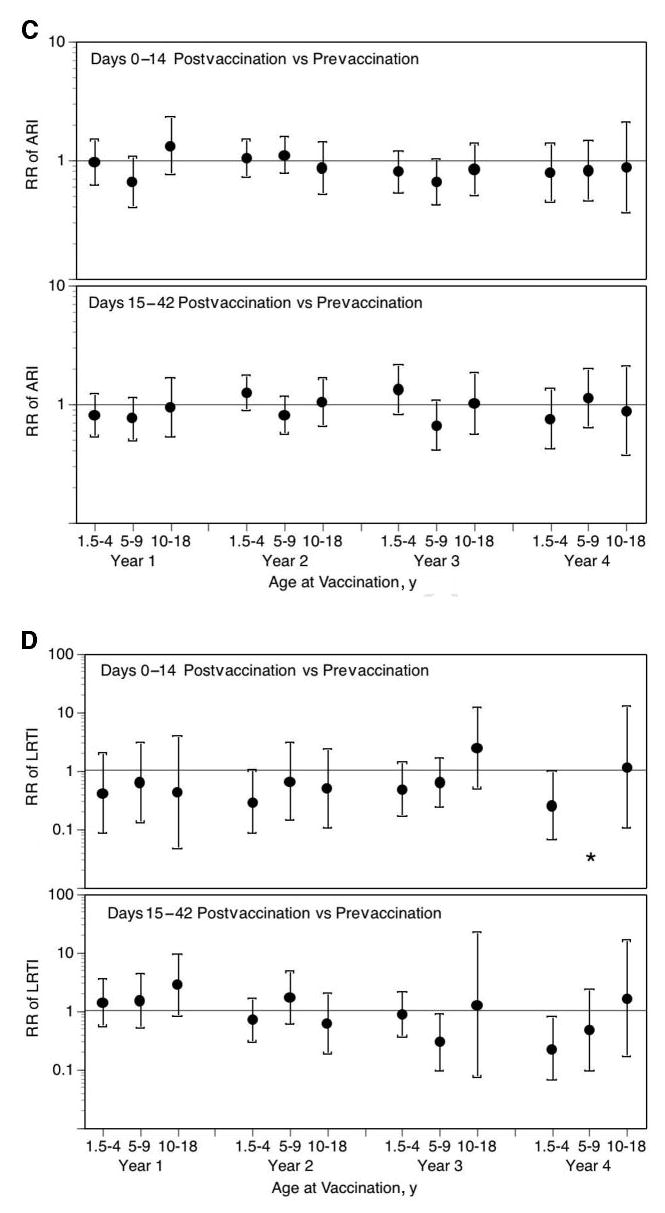
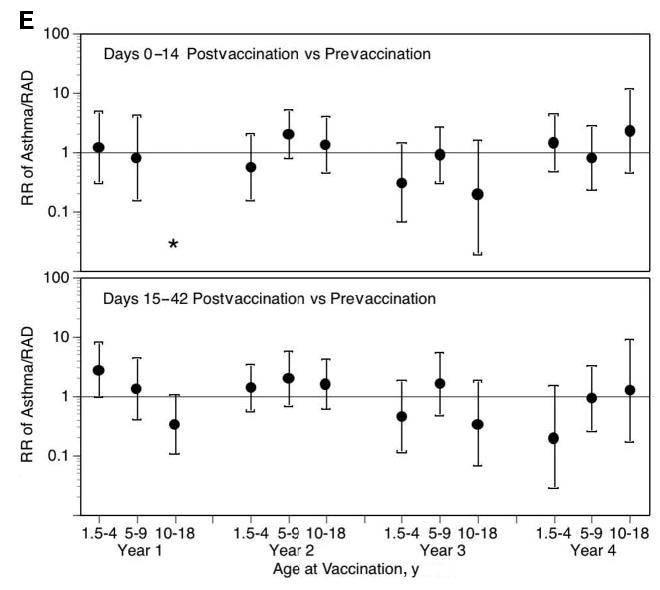
LAIV-T is safe in children who are 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. The relative incidence of MAARI (A), MAARI subcategories (otitis media/sinusitis [B], upper respiratory tract illness [ARI; C], and lower respiratory tract illness [LRTI; D], and asthma/reactive airway disease [asthma/ RAD; E]) 0 to 14 and 15 to 42 days after vaccination was compared with the prevaccination period in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. No significant increase in the risk in health care utilization for MAARI, MAARI subcategories, and asthma was detected in LAIV-T vaccinees 0 to 14 and 15 to 42 days after vaccination except in vaccine year 1. In vaccine year 1, children who were 18 months to 4 years of age had a significantly higher RR (2.85; 95% CI: 1.01–8.03) for asthma events 15 to 42 days after vaccination but not in the subsequent 3 vaccine years.
