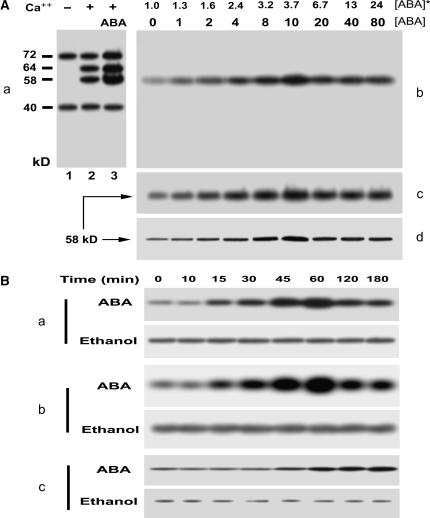
Figure 2.
ABA stimulates the 58-kD Ca2+-dependent protein kinase. A, ABA stimulates the 58-kD Ca2+-dependent protein kinase in a dose-dependent manner. a, ABA-stimulated in vitro phosphorylation in total microsomal proteins. The berry tissues were incubated in a medium (pH 5.5) containing 0 (control) or 10 μm (±)-ABA for 1 h. The microsomes prepared from the tissues were subjected to phosphorylation in vitro and separated by SDS-PAGE, and then phosphoproteins were detected by autoradiography. The detailed procedures of these assays were described in “Materials and Methods.” The phosphoproteins from the control tissues are shown after the in vitro phosphorylation in the absence (lane 1) or presence (lane 2) of Ca2+. The phosphorylation level of some phosphoproteins increases in the microsomes from the ABA-treated tissues after the in vitro phosphorylation in the presence of Ca2+ (lane 3). The calculated molecular masses according to the mobility rate of protein standards are shown at the left of the section in kD; − and + indicate the absence and presence of Ca2+, respectively. b to d, ABA-induced in-gel-stimulation of the 58-kD Ca2+-dependent protein kinase is dose dependent. The microsomal proteins prepared from the ABA-treated tissues were separated by SDS-PAGE and then subjected to in-gel autophosphorylation (b) and histone-phosphorylating activity (c) assays and immunoblotting analysis with rabbit polyclonal antibodies to the CaM-like domain of soybean CDPKα (d). The procedures of the assays were described in “Materials and Methods.” The numbers displayed in line [ABA] indicate the exogenous ABA concentrations applied to the berry tissues, and those in line [ABA]* show the ABA concentrations within the treated tissues determined by radioimmunoassay as described in “Materials and Methods.” The ABA-stimulated in-gel autophosphorylated protein (b) corresponds to the 58-kD phosphoprotein shown in section a. The molecular mass of the kinase phosphorylating histone III-S (c) or that of the signal immunodetected by anti-soybean CDPKα serum (d) is shown to be 58 kD. B, Time course of the ABA-induced stimulation of the 58-kD Ca2+-dependent protein kinase. Berry tissues were incubated for different durations of time from 0 to 180 min in the medium containing 10 μm ABA (ABA) or the same amount of ethanol for solubilizing ABA (Ethanol) as described in “Materials and Methods.” The microsomes prepared from the treated tissues were used for analysis of the 58-kD Ca2+-dependent protein kinase as described in A. a, In-gel autophosphorylation of the 58-kD kinase. b, In-gel histone-phosphorylating activity of the 58-kD kinase. c, 58-kD immunosignal detected by the rabbit antiserum directed against the calmudulin-like domain of soybean CDPKα.