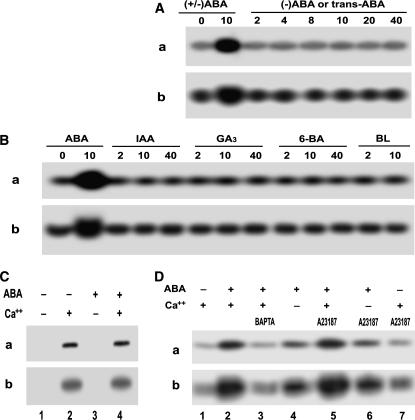
Figure 3.
Some features of ABA-induced ACPK1 kinase stimulation. A, Specificity of the stimulation of the ACPK1 kinase to the physiologically active form of ABA. Berry tissues were incubated in the medium containing various concentrations (indicated by the numbers under lines as μm) of (±)-ABA or ABA isomers for 1 h as described in “Materials and Methods.” In-gel phosphorylation (a) and histone-phosphorylating activity (b) of the 58-kD kinase in the microsomes prepared from the tissues treated by (±)-ABA (as a control) or its isomers (−)-ABA or trans-ABA were analyzed. B, ACPK1 appears to be stimulated uniquely by ABA. In-gel phosphorylation (a) and histone-phosphorylating activity (b) of the 58-kD kinase were assayed in the microsomes prepared from the tissues treated as described in A by (±)-ABA (as a control) or other plant hormones auxin IAA, GA3, synthetic cytokinin N-benzyl-6-aminopurine (6-BA), and brassinolide (BL). The numbers under lines indicate the applied concentrations as μm. C, ABA stimulates ACPK1 kinase indirectly. Microsomes were prepared from nontreated tissues. In-gel phosphorylation (a) and histone-phosphorylating activity (b) of the 58-kD kinase were assayed in the microsomes in the presence or absence of 10 μm ABA or Ca2+ or both; − and + indicate the absence and presence, respectively. D, Effects of apoplasmic Ca2+ and influx of Ca2+ on ABA-induced stimulation of ACPK1 kinase. Berry tissues were preincubated in a medium (the equilibration buffer as detailed in “Materials and Methods”) containing 5 mm BAPTA (a Ca2+ chelator, lane 3) or 5 μm A23187 (a Ca2+ ionophore, lanes 5–7) in the presence of Ca2+ (lanes 3, 5, and 7), or 1 mm EGTA in the absence of Ca2+ (lanes 4 and 6), and then ABA (10 μm except for lane 7 where ABA was absent) was added to the medium for a further incubation of 1 h. The incubation in the presence of Ca2+ but absence of ABA (lane 1) was taken as a control, and that in the presence of both Ca2+ and ABA (lane 2) as another control. The microsomes were prepared from the treated tissues. In-gel autophosphorylation (a) and histone-phosphorylating activity (b) of the 58-kD kinase in the microsomes were analyzed in the presence of Ca2+; − and + indicate the absence and presence, respectively.