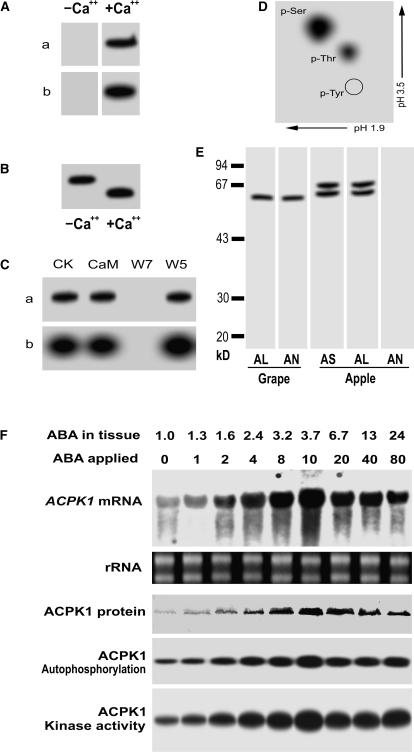
Figure 7.
Characterization of the products of ACPK1 gene and up-regulation of both its products and transcripts by ABA. A, The purified recombinant protein (10 μg) of the full-length ACPK1 (ACPK1L) was subjected to SDS-PAGE and then assayed for its in-gel autophosphorylation (a) and histone-phosphorylating activity (b) in the absence (−Ca2+) or presence (+Ca2+) of Ca2+. B, Ca2+-dependent electrophoretic mobility shift of the ACPK1L, shown by its in-gel autophosphorylation; −Ca2+ and +Ca2+ indicate the absence and presence of Ca2+, respectively. C, Neither the in-gel autophosphorylation (a) nor histone-phosphorylating activity (b) is activated by CaM, whereas both activities are inhibited by CaM antagonist W7 but not by W5. CK, Control. D, Phosphoamino acid analysis of the autophosphorylated ACPK1L. γ-32P-labeled ACPK1L was hydrolyzed with HCl and subjected to two-dimensional thin-layer electrophoresis. The positions of phospho-Ser (p-Ser), phospho-Thr (p-Thr), and phospho-Tyr (p-Tyr) are indicated. E, Specificity of the anti-ACPK1-N40 serum to ACPK1. The 58-kD ACPK1 immunosignal was detected in the microsomes of grape mesocarp (Grape) by both the anti-ACPK1L (indicated by AL) and -ACPK1-N40 (indicated by AN) sera. In the microsomes of apple flesh prepared with the same procedures as described in “Materials and Methods” for grape mesocarp, two immunosignals having molecular mass of about 60 and 67 kD, respectively, were detected by the antiserum against CaM-like domain of soybean CDPKα (indicated by AS) or anti-ACPK1L serum (AL), but not by anti-ACPK1-N40 serum (AN). F, ABA up-regulates gene expression, autophosphorylation, and kinase activity of ACPK1. Berry tissues were incubated in the medium containing different concentrations of (±)-ABA from 0 to 80 μm (indicated by ABA applied; unit: μm), and after the incubation, the concentrations of ABA present in the treated tissues (ABA in tissue; unit: μm) were determined by radioimmunoassay as described in “Materials and Methods.” Total RNA and microsomes were extracted from the treated tissues, respectively. Total RNA (20 μg), transferred on a nylon membrane after electrophoresed in an agarose gel, was hybridized with the 32P-labeled cDNA fragment corresponding to the N-terminal variable domain of ACPK1 to detect specifically mRNA level of ACPK1 (ACPK1 mRNA). rRNA indicates loading control of the RNA samples stained with ethidium bromide. Total microsomal proteins (50 μg) were immunoprecipitated with 3 μg anti-ACPK1-N40 serum protein. The immunoprecipitated proteins were subjected to SDS-PAGE and then to the assays for detecting the levels of ACPK1 protein (ACPK1 protein, blotted with anti-ACPK1-N40 serum), autophosphorylation (ACPK1 Autophosphorylation), or histone-phosphorylating kinase activity (ACPK1 Kinase activity) as described in “Materials and Methods.”