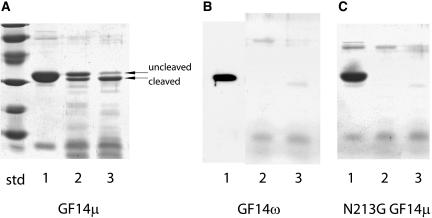
Figure 5.
Proteolytic analysis of GF14μ, GF14ω, and N213G GF14μ mutant structures demonstrates increased flexibility for the medial Gly containing divalent cation-binding domain. Aliquots of purified, bacterially expressed GF14μ (A), GF14ω (B), and N213G GF14μ (C) were digested at 25°C with endoproteinase Lys-C at an enzyme to substrate ratio of 1:100 in the presence (A, B, and C, lane 2) or absence (A, B, and C, lane 3) of 5 mm CaCl2. Denatured samples of 0 h digest (A, B, and C, lane 1), 4 h digest with CaCl2 (A, B, and C, lane 2), and 4 h digest without CaCl2 (A, B, and C, lane 3) were electrophoresed and visualized with Coomassie stain. Uncleaved and cleaved proteins are indicated. Divalent cation induced structural changes were observed; however, replacement of Gly for Asn in the divalent cation-binding loop increased sensitivity to proteolytic activity suggesting increased flexibility.