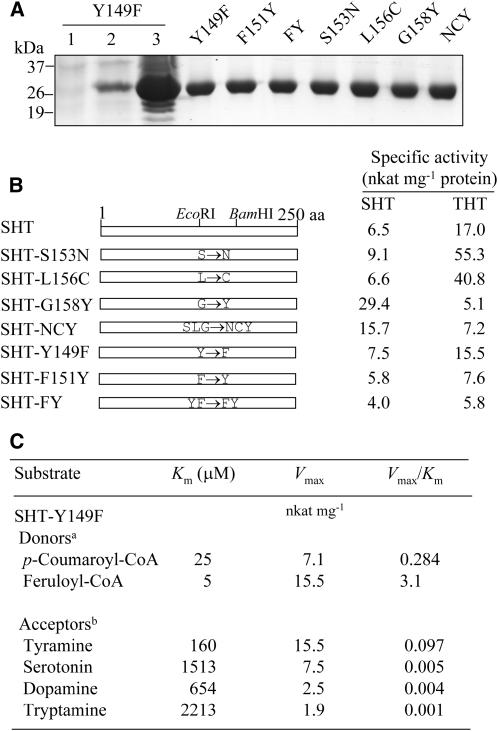
Figure 7.
Specific activities of point-mutated SHT proteins and kinetic analysis of SHT-Y149F protein. A, Expression of the mutated SHT genes in E. coli and affinity purification of His-tagged mutated SHT constructs. Lane 1, Total proteins in 10 μL of bacterial cells of the SHT-Y149F strain grown without IPTG; lane 2, total proteins in 10 μL of bacterial cells of the SHT-Y149F strain after IPTG treatment; lane 3, 20 μg of soluble proteins from the SHT-Y149F strain; right lanes (Y149F through NCY), mutated SHT proteins (10 μg) purified by affinity (Ni-NTA) chromatography. B, Constructs of point-mutated SHT chimeras and measurements of their specific activities. SHT-FY is a SHT protein containing two mutated residues in which Tyr-149 and Phe-151 were replaced with the corresponding Phe-145 and Tyr-147 of THT. SHT-NCY is a SHT protein containing three mutated residues in which Ser-153, Leu-156, and Gly-158 were replaced with the corresponding Asn-149, Cys-152, and Tyr-154 of THT, respectively. Enzyme assays were performed as described above. C, Substrate specificity analysis of the SHT-Y149F protein. Footnote a, Tyramine (1 mm) was used as the acyl acceptor. Footnote b, Feruloyl-CoA (250 μm) was used as the acyl donor.