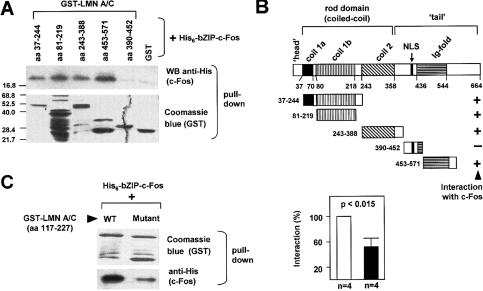
Figure 1.
The interaction between lamin A/C and c-Fos is mediated by leucine heptad repeats. (A) GST fusion proteins spanning different regions of rat lamin A/C (LMN A/C) were tested for their interaction with hexahistidine-tagged c-Fos basic-leucine-zipper (His6-bZIPc-Fos, rat amino acids 116–211). Protein complexes were precipitated using glutathione-sepharose 4B and washed extensively. The presence of His6-bZIPc-Fos and GST proteins in the precipitated material was monitored using anti-His antibody and Coomassie blue staining, respectively. (B) Summary of pull-down experiments showing the domains of rat lamin A/C. (NLS) Nuclear localization signal; (+) positive interaction; (-) negative interaction. (C) Pull-down assay using His6-bZIPc-Fos and GST fused to the murine lamin A/C region containing a portion of coil 1 (amino acids 117–227), either wild-type (WT) or the mutant sequence L158G-E159T-L172S-E173T (Mutant) in which one “leucine heptad repeat” has been disrupted (see Supplementary Fig. S1C). (Left) Representative example showing the amount of GST-lamin A/C 117–227 (wild type [WT] and Mutant) and His6-bZIPc-Fos in the precipitate. (Right) Quantification of the interaction as determined by densitometric analysis of four independent experiments (wild type [WT] set as 100%).