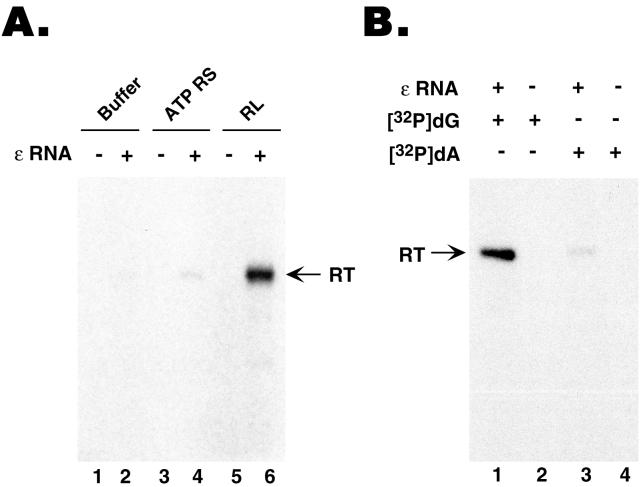
FIG. 4.
GST-MiniRT2 purified from mammalian cells was active in the initiation of protein priming but defective in DNA polymerization. GST-MiniRT2 was expressed in 293T cells following transient transfection of pEBG-MiniRT2 and purified using GSH affinity resin. (A) Purified GST-MiniRT2 was assayed for in vitro protein priming activity, supplemented with buffer alone (lanes 1 and 2), with an ATP regenerating system (ATP RS [lanes 3 and 4), or with reticulocyte lysate (RL [lanes 5 and 6]). The ɛ RNA was added to the indicated reaction mixtures only (lanes 2, 4, and 6). [α-32P]dGTP was used as the nucleotide precursor. (B) Purified GST-MiniRT2 was assayed for in vitro protein priming, all reactions being supplemented with reticulocyte lysate. Either [α-32P]dGTP (lanes 1 and 2) or [α-32P]dATP (plus unlabeled dGTP and TTP) (lanes 3 and 4) was used as the labeled nucleotide precursor. The ɛ RNA was added to the mixtures for reactions 1 and 3 only.