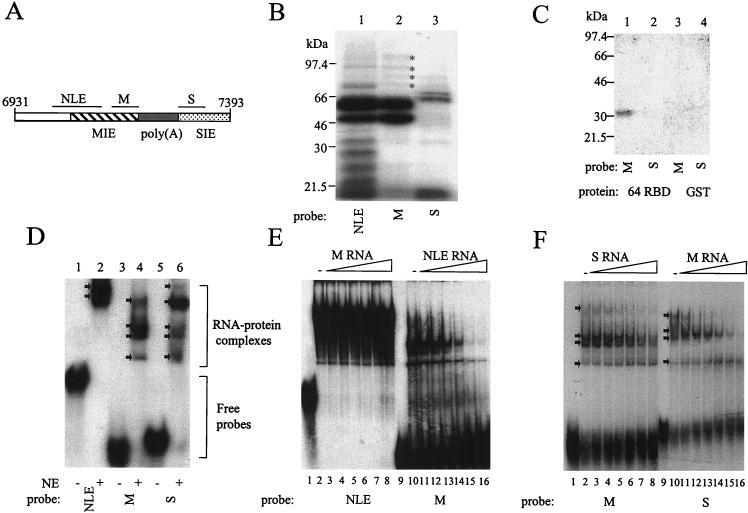
FIG. 7.
UV cross-linking and EMSA of protein binding to sequences surrounding the late poly(A) signal. (A) Diagram of the HPV-31 late gene 3′ UTR sequences showing the positions of the probes used for protein binding studies. Hatched box, MIE; grey box, poly(A) and CstF binding sites; stippled box, SIE; lines, positions of NLE probe (nt 7041 to 7141), M probe (nt 7161 to 7211), and S probe (nt 7284 to 7343). (B) UV cross-linking of 32P-labeled NLE, M, and S probes to HeLa nuclear extracts. Lane 1, NLE (101 nt); lane 2, M (50 nt); lane 3, S (60 nt). Asterisks, minor proteins of similar sizes that bind both NLE and M probes. (C) UV cross-linking of 32P-labeled M and S probes to bacterially expressed CstF-64 RBD. Lane 1, GST-tagged CstF-64 RBD protein with an M probe; lane 2, GST-tagged CstF-64 RBD protein with an S probe; lane 3, GST protein with an M probe; lane 4, GST protein with an S probe. 64 RBD, GST-tagged CstF-64 RBD. (D) EMSA of 32P-labeled NLE, M, and S probes using HeLa cell nuclear extracts. RNA-protein complexes and free probes are bracketed. Arrows, RNA-protein complexes; NE, HeLa nuclear extracts. (E) EMSA competition assay. Lanes 1 to 8, 32P-labeled NLE probe (1.5 pmol); lane 1, no extracts; lane 2, no competitor RNA; lanes 3 to 8, 1- to 32-fold molar excess, i.e., 1.5 to 48 pmol of in vitro-transcribed unlabeled M RNA; lanes 9 to 16, 32P-labeled M probe (1.5 pmol); lane 9, no extracts; lane 10, no competitor RNA; lanes 11 to 16, 1- to 32-fold molar excess, i.e., 1.5 to 48 pmol of in vitro-transcribed unlabeled NLE RNA. (F) EMSA competition assay. Lanes 1 to 8, 32P-labeled M probe (1.5 pmol); lane 1, no extracts; lane 2, no competitor RNA; lanes 3 to 6, 1- to 32-fold molar excess, i.e., 1.5 to 48 pmol of in vitro-transcribed unlabeled S RNA; lanes 9 to 16, 32P-labeled S probe (1.5 pmol); lane 9, no extracts; lane 10, no competitor RNA; lanes 11 to 16, 1- to 32-fold molar excess, i.e., 1.5 to 48 pmol of in vitro-transcribed, unlabeled M RNA.