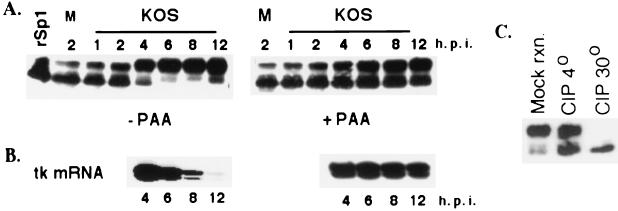
FIG. 1.
Modification of Sp1 during HSV-1 infection. (A) Western blot analysis of cell extracts prepared from Vero cells infected with KOS in the presence and absence of PAA. At indicated times after infection, the infected cells were harvested directly into the SDS sample buffer, resolved on an SDS-8% acrylamide gel, and transferred to a polyvinylidene difluoride membrane. The membrane was immunoblotted with Sp1-specific antibody. In the first lane, purified baculovirus-produced Sp1 (rSp1) was run alongside the samples as a control. M designates the sample from the mock infection. (B) Primer extension analysis using total RNA from KOS-infected cells in the presence and absence of PAA. RNA was isolated at the indicated times, and 6 μg of total RNA from each sample was used for primer extension reaction using a tk-specific primer. The products were resolved on a 6% sequencing gel, dried, and exposed to film for autoradiography. (C) Phosphatase treatment of infected cell Sp1. Lysate from Vero cells infected with HSV for 8 h (∼100 μg) was incubated in a reaction mixture containing 10 mM (each) sodium fluoride and sodium phosphate at 30o (mock reaction [mock rxn.]), 10 U of CIP at 4o (CIP 4o), and 10 U of CIP at 30o (CIP 30o). All reactions were performed for 10 min in 50 mM Tris-HCl (pH 7.5)-1.0 mM MgCl2. Following the reactions, the samples were subjected to Western blot analysis as described for panel A.