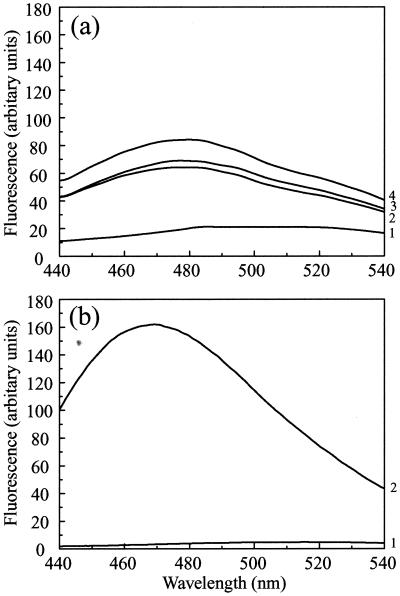
FIG. 7.
ANS binding. (a) ANS (at a final concentration of 20 μM) was added to purified pre-VP22a scaffold particles (at 0.2 mg/ml) after they had been dialyzed against PBS containing 1 mM DTT that had been adjusted to pH 7.2 (trace 1), pH 6 (trace 2), or pH 5.5 (trace 3) as described in the text. The excitation wavelength was 370 nm, and fluorescence was recorded at between 440 and 540 nm. A control spectrum of ANS in PBS (pH 7.2) was measured (trace 4); there was no significant difference between the fluorescence of free ANS over the range from pH 5.5 to pH 7.2. (b) For comparative purposes, the ANS binding spectrum of the capsid shell protein VP23, which forms a molten globule, is reproduced from reference 23. Only the curves for ANS (20 μM) alone (trace 1) or mixed with VP23 (0.2 mg/ml) (trace 2) are shown here. The spectra in panel b were recorded using lower slit widths than for those in panel a, resulting in an approximately fourfold decrease in the intensities in panel b compared with panel a, as shown by comparing the spectra for 20 μM ANS in the two panels. When this factor is taken into account, it is clear that the enhancement of ANS fluorescence in the case of VP23 (b) is at least eightfold higher than that in the case of pre-VP22a at pH 5.5 (a).