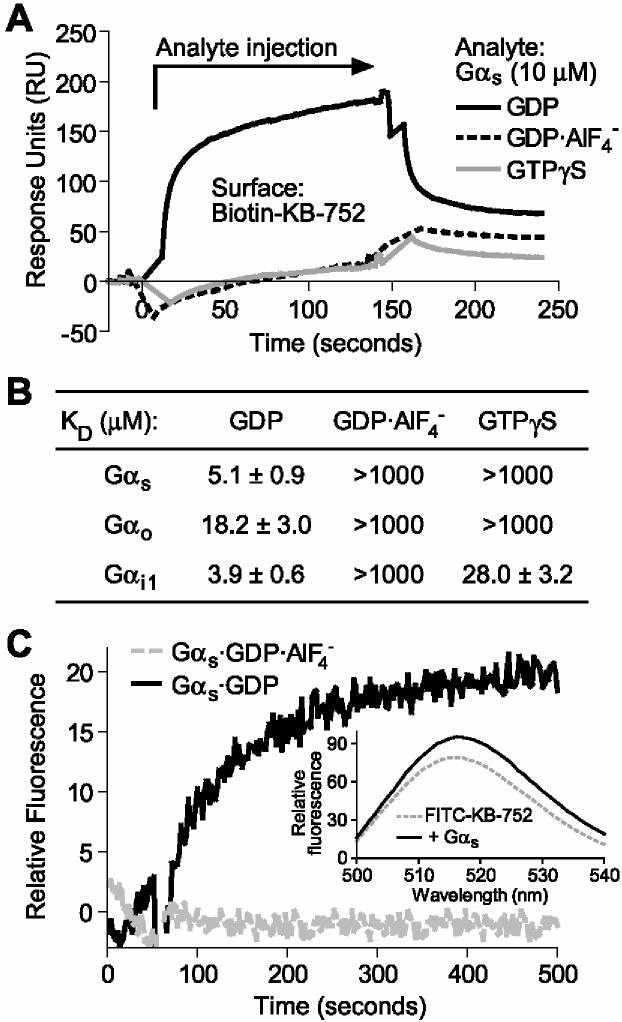
Figure 1.
Nucleotide-dependent binding of KB-752 to Gαs. (A) 10 μM of Gαs (“Analyte”), in each of the indicated nucleotide bound states, was injected over immobilized KB-752 and binding measured by SPR. Non-specific binding to a control biotinylated peptide surface was subtracted from each curve. (B) Gαs, Gαo, and Gαi1 were separately injected at increasing concentrations (0.01 to 50 μM) over immobilized KB-752 to determine the dissociation constants (KD) for each interaction pair as obtained from simultaneous kon/koff analyses (n = 4-6 for each state). (C) Time course of FITC-KB-752 binding to Gαs. 100 nM FITC-KB-752 was equilibrated in buffer containing either GDP (black trace) or GDP·AlF4- (grey trace) and background fluorescence subtracted. At 50 seconds, 1 μM Gαs·GDP (black trace) or 1 μM Gα·GDP·AlF4- (grey trace) was added. (Inset) Emission scans of FITC-KB-752 in the absence and presence of Gαs·GDP obtained using an excitation wavelength of 440 nm.