Abstract
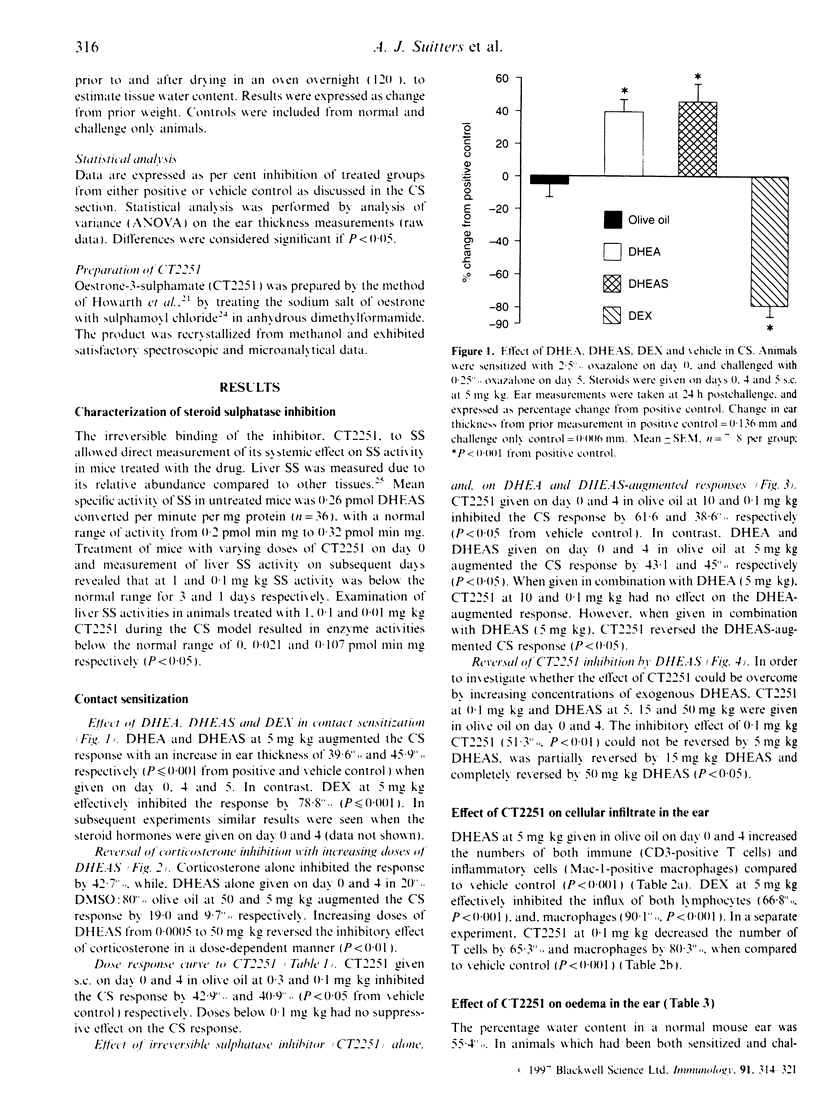
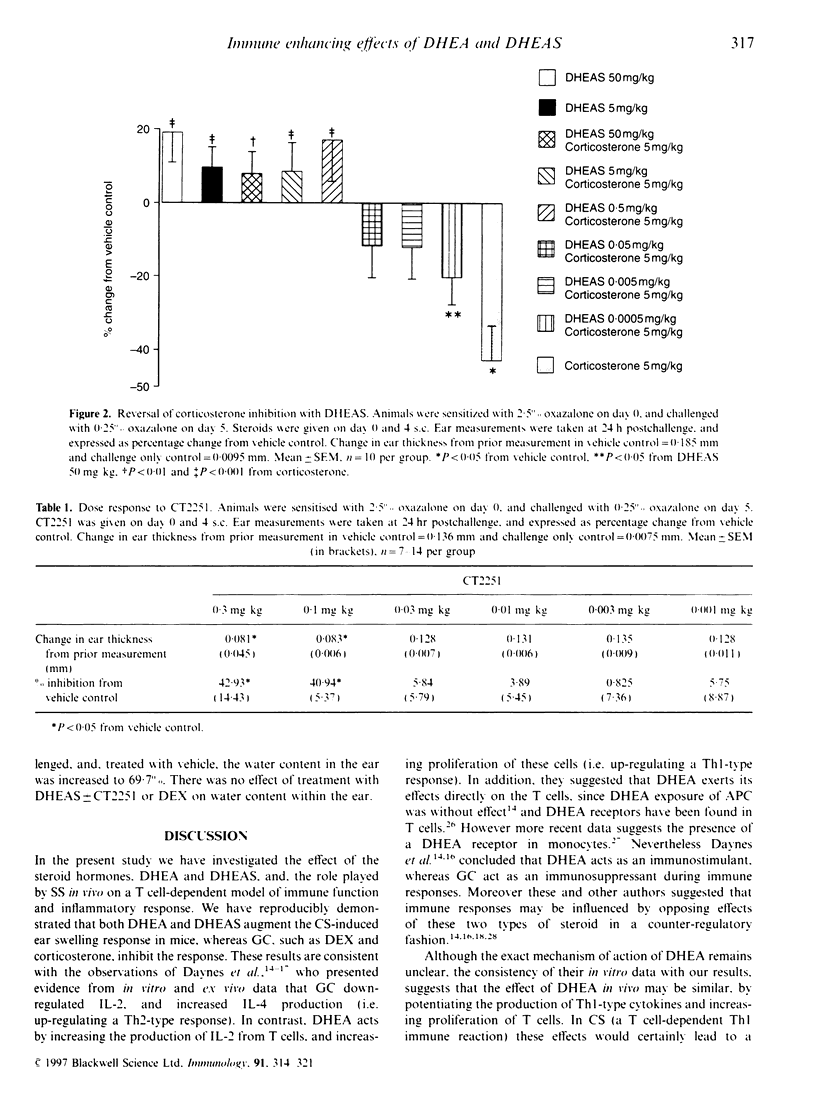
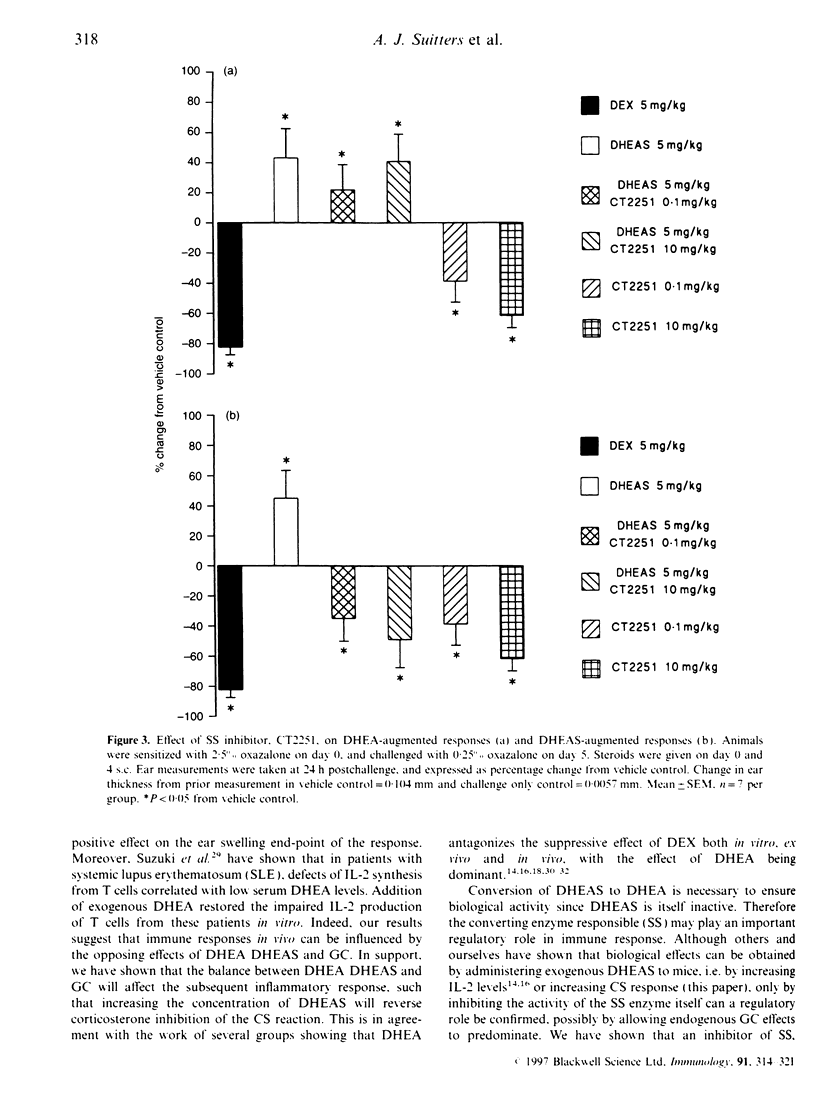
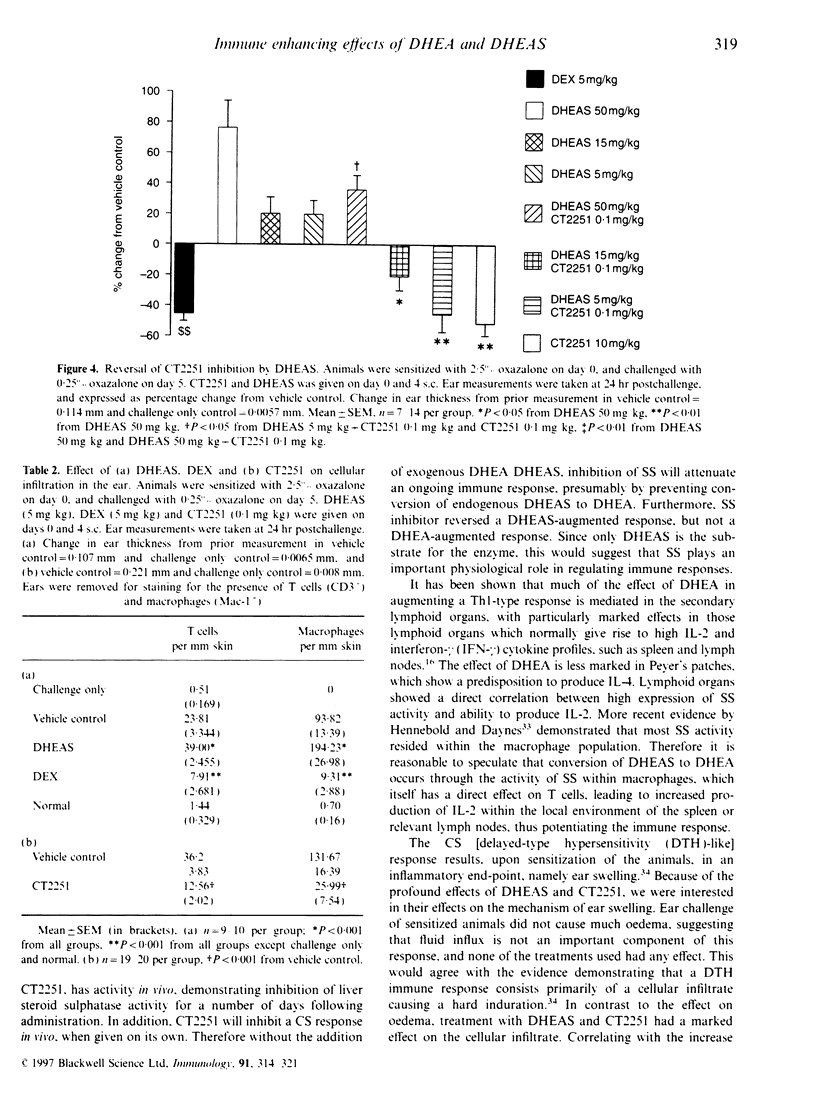
Steroid hormones, such as glucocorticoids (GC), influence immune and inflammatory responses through their suppressive actions. Recent evidence suggests that another steroid hormone, dehydroepiandrosterone (DHEA), provides an immunostimulatory influence opposing the effect of GC. DHEA circulates in its inactive sulphated form, DHEAS, requiring conversion to DHEA by a steroid sulphatase (SS) enzyme for biological activity. Therefore, inhibition of SS activity may affect immune responses, allowing endogenous GC effects to predominate. We have shown that administration of DHEA and DHEAS in contact sensitization (CS) augments ear swelling by 39 and 46% respectively (P < 0.001). DHEAS at doses of 0.5, 5 and 50 mg/kg reverses the inhibitory effect of corticosterone (5 mg/kg) (P < 0.01). In CS, CT2251 (SS inhibitor) at 10 and 0.1 mg/kg inhibited ear swelling by 61 and 38% (P < 0.05) respectively. In addition, it inhibited DHEAS-augmented responses by 49 and 35% respectively (P < 0.05), with no effect on DHEA-augmented responses. DHEAS reversed CT2251 inhibition of the CS response with complete reversal at 50 mg/kg (P < 0.05). DHEAS and CT2251 appear to affect cellular infiltration into the ear, since DHEAS increased the number of lymphocytes by 63.8% and macrophages by 107% (P < 0.001), whereas CT2251 at 0.1 mg/kg decreased the number of lymphocytes by 65% (P < 0.001) and macrophages by 80% (P < 0.001). DHEAS, CT2251 and dexamethasone had no effect on oedema in the ear. From our data we have shown that steroid hormones, such as DHEA, have the potential to act as immunostimulatory factors in vivo. Inhibiting the conversion of DHEAS to DHEA by SS enzyme leads to an anti-inflammatory effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araneo B., Daynes R. Dehydroepiandrosterone functions as more than an antiglucocorticoid in preserving immunocompetence after thermal injury. Endocrinology. 1995 Feb;136(2):393–401. doi: 10.1210/endo.136.2.7835270. [DOI] [PubMed] [Google Scholar]
- Blauer K. L., Poth M., Rogers W. M., Bernton E. W. Dehydroepiandrosterone antagonizes the suppressive effects of dexamethasone on lymphocyte proliferation. Endocrinology. 1991 Dec;129(6):3174–3179. doi: 10.1210/endo-129-6-3174. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Browne E. S., Wright B. E., Porter J. R., Svec F. Dehydroepiandrosterone: antiglucocorticoid action in mice. Am J Med Sci. 1992 Jun;303(6):366–371. doi: 10.1097/00000441-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Leiter E. H., Schwizer R. W. Therapeutic effects of dehydroepiandrosterone (DHEA) in diabetic mice. Diabetes. 1982 Sep;31(9):830–833. doi: 10.2337/diab.31.9.830. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Dudley D. J., Araneo B. A. Regulation of murine lymphokine production in vivo. II. Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells. Eur J Immunol. 1990 Apr;20(4):793–802. doi: 10.1002/eji.1830200413. [DOI] [PubMed] [Google Scholar]
- Hobkirk R. Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol. 1985 Nov;63(11):1127–1144. doi: 10.1139/o85-141. [DOI] [PubMed] [Google Scholar]
- Howarth N. M., Purohit A., Reed M. J., Potter B. V. Estrone sulfamates: potent inhibitors of estrone sulfatase with therapeutic potential. J Med Chem. 1994 Jan 21;37(2):219–221. doi: 10.1021/jm00028a002. [DOI] [PubMed] [Google Scholar]
- Liu C. H., Laughlin G. A., Fischer U. G., Yen S. S. Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in postmenopausal women: evidence for a reduced 17,20-desmolase enzymatic activity. J Clin Endocrinol Metab. 1990 Oct;71(4):900–906. doi: 10.1210/jcem-71-4-900. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Banks J. INHIBITION OF MAMMALIAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE BY STEROIDS. Proc Natl Acad Sci U S A. 1960 Apr;46(4):447–452. doi: 10.1073/pnas.46.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan J. A., Serkin C. D., Bakouche O. Dehydroepiandrosterone modulation of lipopolysaccharide-stimulated monocyte cytotoxicity. J Immunol. 1996 Jan 1;156(1):328–335. [PubMed] [Google Scholar]
- Meikle A. W., Daynes R. A., Araneo B. A. Adrenal androgen secretion and biologic effects. Endocrinol Metab Clin North Am. 1991 Jun;20(2):381–400. [PubMed] [Google Scholar]
- Meikle A. W., Dorchuck R. W., Araneo B. A., Stringham J. D., Evans T. G., Spruance S. L., Daynes R. A. The presence of a dehydroepiandrosterone-specific receptor binding complex in murine T cells. J Steroid Biochem Mol Biol. 1992 May;42(3-4):293–304. doi: 10.1016/0960-0760(92)90132-3. [DOI] [PubMed] [Google Scholar]
- Orentreich N., Brind J. L., Rizer R. L., Vogelman J. H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984 Sep;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Padgett D. A., Loria R. M. In vitro potentiation of lymphocyte activation by dehydroepiandrosterone, androstenediol, and androstenetriol. J Immunol. 1994 Aug 15;153(4):1544–1552. [PubMed] [Google Scholar]
- Punjabi U., Deslypere J. P., Verdonck L., Vermeulen A. Androgen and precursor levels in serum and testes of adult rats under basal conditions and after hCG stimulation. J Steroid Biochem. 1983 Oct;19(4):1481–1490. doi: 10.1016/0022-4731(83)91124-x. [DOI] [PubMed] [Google Scholar]
- Purohit A., Williams G. J., Howarth N. M., Potter B. V., Reed M. J. Inactivation of steroid sulfatase by an active site-directed inhibitor, estrone-3-O-sulfamate. Biochemistry. 1995 Sep 12;34(36):11508–11514. doi: 10.1021/bi00036a025. [DOI] [PubMed] [Google Scholar]
- Regelson W., Loria R., Kalimi M. Hormonal intervention: "buffer hormones" or "state dependency". The role of dehydroepiandrosterone (DHEA), thyroid hormone, estrogen and hypophysectomy in aging. Ann N Y Acad Sci. 1988;521:260–273. doi: 10.1111/j.1749-6632.1988.tb35284.x. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Hernandez-Pando R., Lightman S. L. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994 Jul;15(7):301–303. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Suzuki N., Daynes R. A., Engleman E. G. Dehydroepiandrosterone enhances IL2 production and cytotoxic effector function of human T cells. Clin Immunol Immunopathol. 1991 Nov;61(2 Pt 1):202–211. doi: 10.1016/s0090-1229(05)80024-8. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Suzuki N., Engleman E. G., Mizushima Y., Sakane T. Low serum levels of dehydroepiandrosterone may cause deficient IL-2 production by lymphocytes in patients with systemic lupus erythematosus (SLE). Clin Exp Immunol. 1995 Feb;99(2):251–255. doi: 10.1111/j.1365-2249.1995.tb05541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann U., Wichmann G., Krause W. Serum levels of testosterone precursors, testosterone and estradiol in 10 animal species. Exp Clin Endocrinol. 1984 May;83(3):283–290. doi: 10.1055/s-0029-1210342. [DOI] [PubMed] [Google Scholar]


