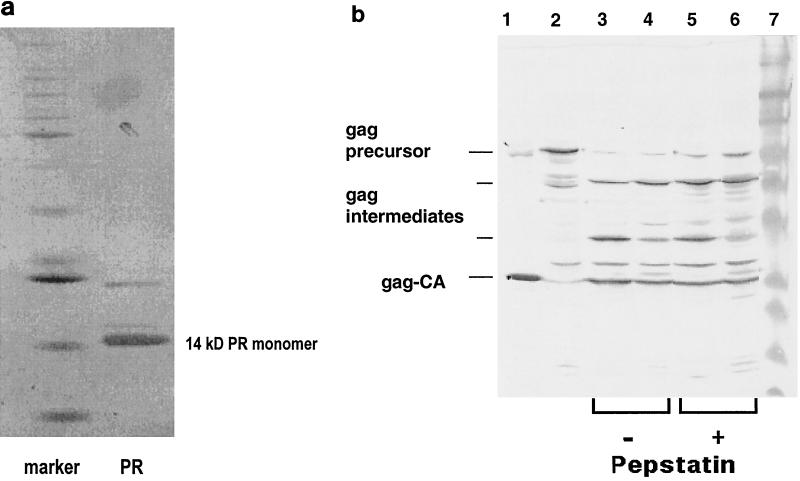
FIG. 3.
(a) Enzyme purification. After purification by cation and HIC column chromatography, the recombinant protease (PR) was analyzed by silver-stained SDS-PAGE. The marker lane contained commercial standard protein size markers (12 to 85 kDa). (b) Protease activity. Shown are the recombinant Gag precursor alone (lane 2) and its cleavage products after incubation at 37°C for 20 min with the recombinant PERV protease (lanes 3 to 6). The reaction mixture was separated by SDS-PAGE and was then Western blotted and reacted with antibody against PERV Gag (polyclonal rabbit anti-PERV GagCA serum [produced in collaboration with Charles River, Kisslegg, Germany] and a secondary goat anti-rabbit immunoglobulin G antiserum coupled to horseradish peroxidase [Dako, Hamburg, Germany]). Shown are cleavage of the recombinant Gag precursor by recombinant PERV protease subtype A/B (lane 3) and subtype C (lane 4) and cleavage in presence of 0.1 mM pepstatin A by PERV A/B (lane 5) or PERV C (lane 6). Purified PERV subtype A/B virus served as a marker (lane 1); lane 7 is a standard protein size marker.