Abstract
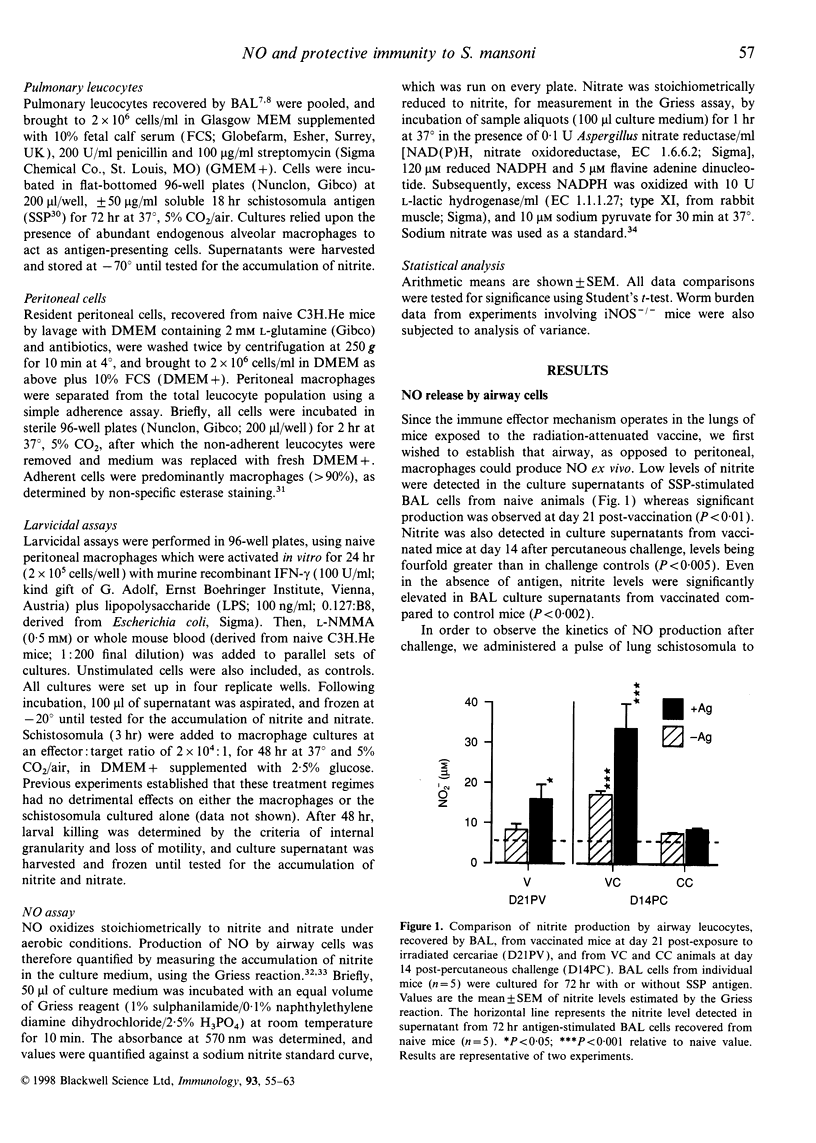
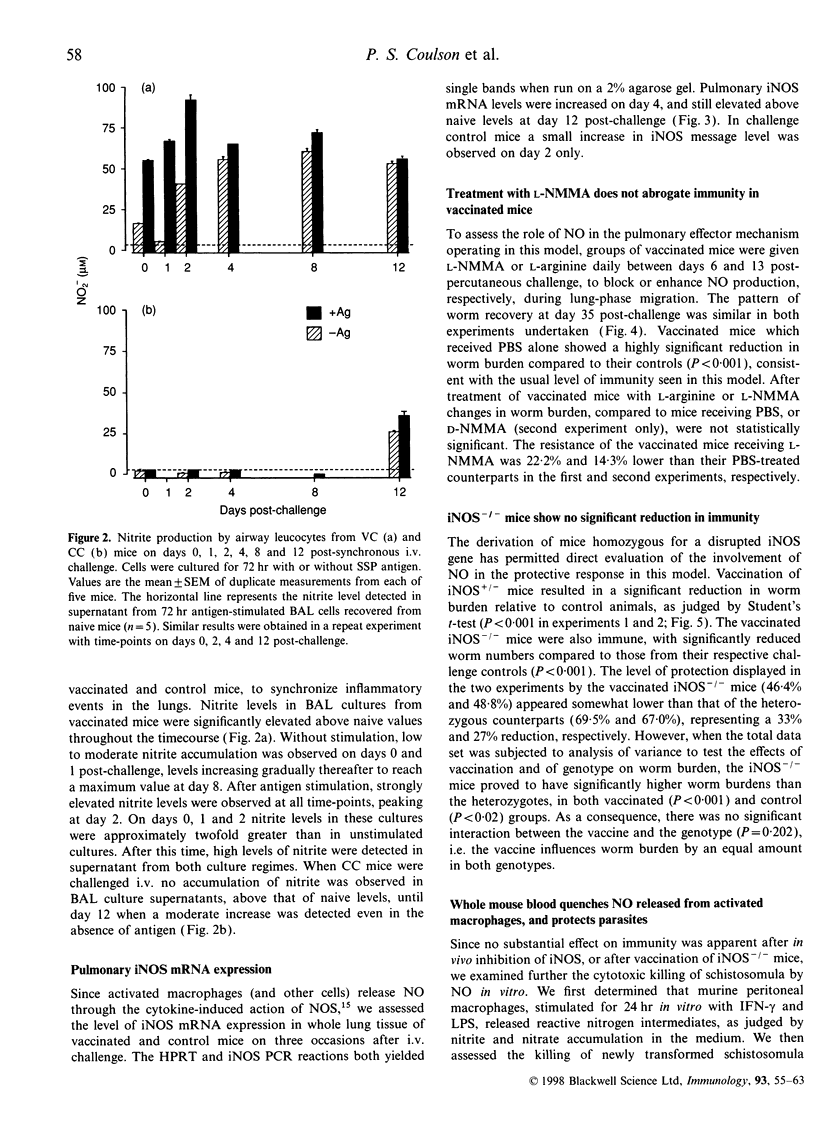
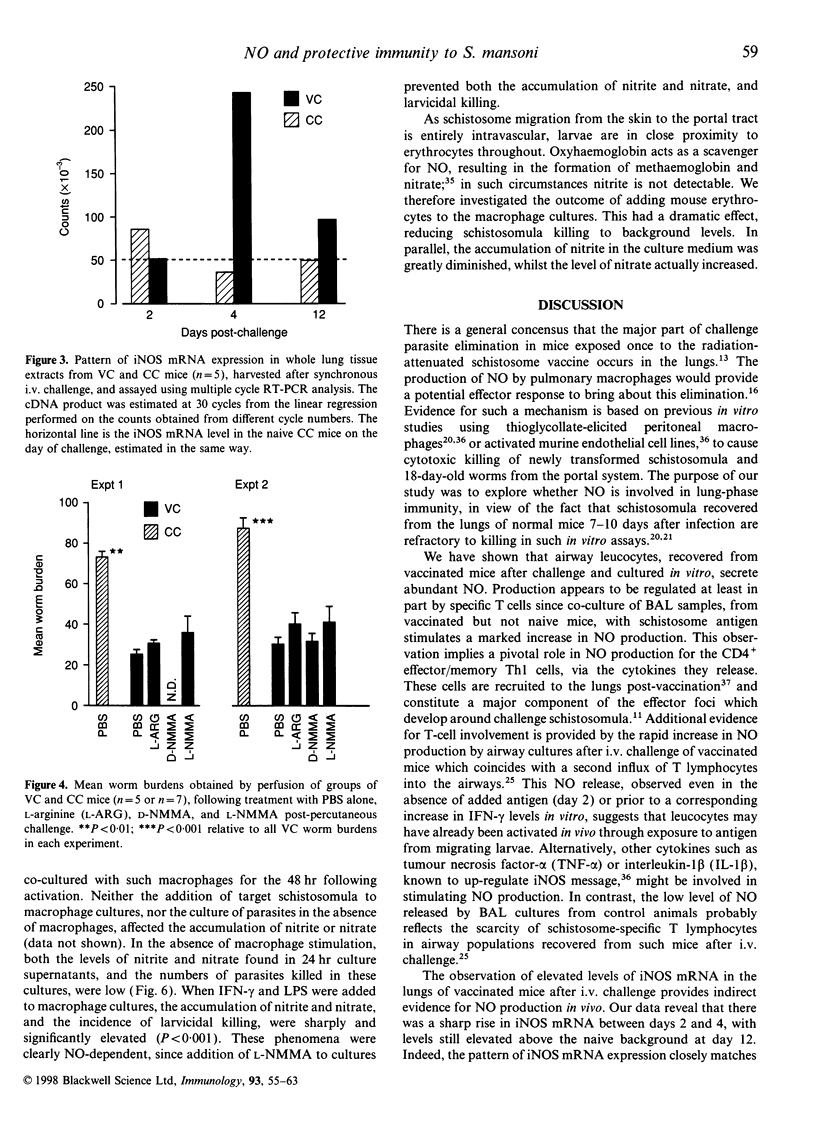
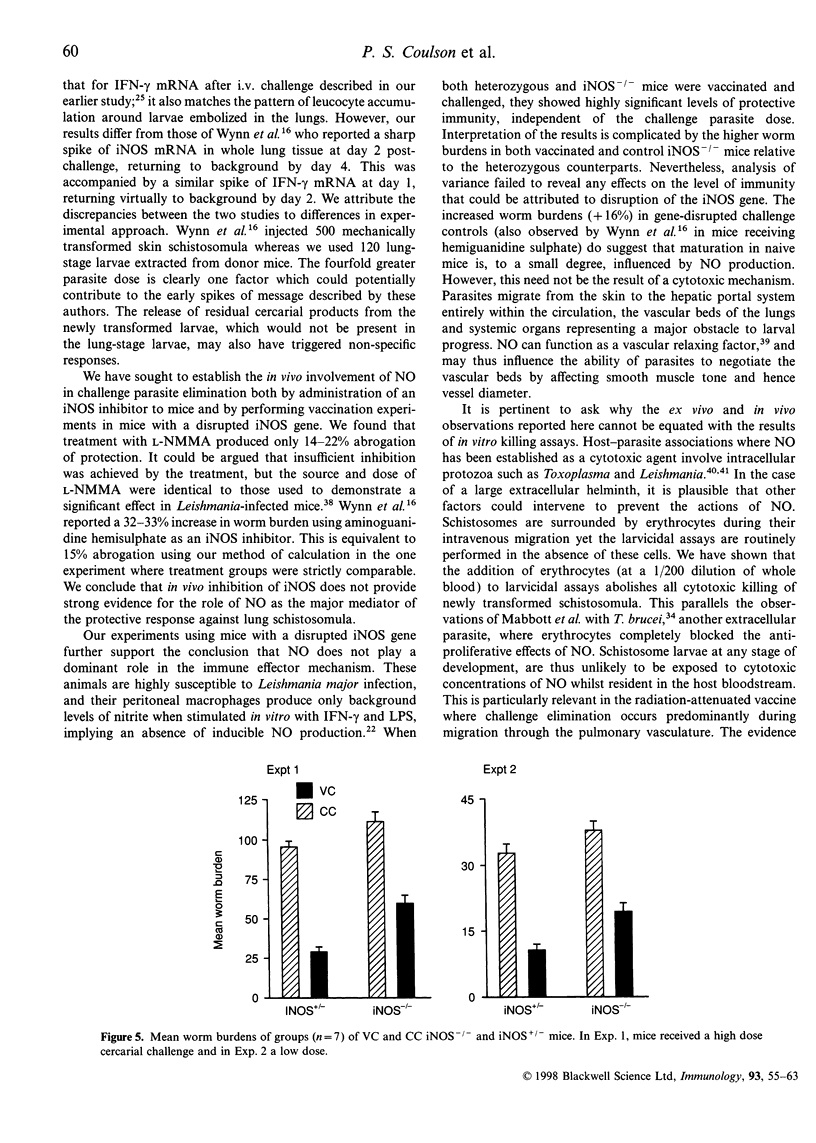
Mice vaccinated with radiation-attenuated cercariae of Schistosoma mansoni exhibit high levels of protection against a challenge with normal larvae. The immune effector mechanism, which operates against schistosomula in the lungs, requires CD4+ T cells capable of producing interferon-gamma (IFN-gamma). This cytokine can stimulate production of nitric oxide (NO), via its ability to up-regulate inducible nitric oxide synthase (iNOS). We have therefore evaluated the potential role of NO in the effector mechanism operating in vaccinated mice. Evidence for the production of NO in the lungs of such animals was obtained from assays on antigen-stimulated airway cell cultures. Enhanced levels of NO, compared with those in cultures from control mice, were detected both after vaccination and after challenge; elevated levels of iNOS mRNA were also present in whole lung after challenge. However, administration of an iNOS inhibitor to vaccinated mice after percutaneous challenge did not significantly increase the worm burden. Furthermore, when mice with a disrupted iNOS gene were vaccinated they showed a highly significant level of protection. Although NO from activated macrophages can mediate cytotoxic killing of newly transformed schistosomula in vitro, we have demonstrated that the addition of erythrocytes to these larvicidal assays abolishes its effects. We interpret this to mean that once migrating schistosomula enter the bloodstream they will be protected against the cytotoxic actions of NO. Our data thus provide little evidence to implicate NO as a major component of the pulmonary effector response to S. mansoni in vaccinated mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. F., Oswald I. P., Caspar P., Hieny S., Keefer L., Sher A., James S. L. Developmental differences determine larval susceptibility to nitric oxide-mediated killing in a murine model of vaccination against Schistosoma mansoni. Infect Immun. 1997 Jan;65(1):219–226. doi: 10.1128/iai.65.1.219-226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson P. S., Wilson R. A. Examination of the mechanisms of pulmonary phase resistance to Schistosoma mansoni in vaccinated mice. Am J Trop Med Hyg. 1988 May;38(3):529–539. doi: 10.4269/ajtmh.1988.38.529. [DOI] [PubMed] [Google Scholar]
- Coulson P. S., Wilson R. A. Pulmonary T helper lymphocytes are CD44hi, CD45RB- effector/memory cells in mice vaccinated with attenuated cercariae of Schistosoma mansoni. J Immunol. 1993 Oct 1;151(7):3663–3671. [PubMed] [Google Scholar]
- Crabtree J. E., Wilson R. A. The role of pulmonary cellular reactions in the resistance of vaccinated mice to Schistosoma mansoni. Parasite Immunol. 1986 May;8(3):265–285. doi: 10.1111/j.1365-3024.1986.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Montgomery R. A., Larsen C. P., Wanders A., Wells A. F. Cytokine gene expression: analysis using northern blotting, polymerase chain reaction and in situ hybridization. Immunol Rev. 1991 Feb;119:163–179. doi: 10.1111/j.1600-065x.1991.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Dean D. A. Schistosoma and related genera: acquired resistance in mice. Exp Parasitol. 1983 Feb;55(1):1–104. doi: 10.1016/0014-4894(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Ennist D. L., Jones K. H. Rapid method for identification of macrophages in suspension by acid alpha-naphthyl acetate esterase activity. J Histochem Cytochem. 1983 Jul;31(7):960–963. doi: 10.1177/31.7.6189884. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., James S. L., Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Kambara T., Wilson R. A. In situ pulmonary responses of T cell and macrophage subpopulations to a challenge infection in mice vaccinated with irradiated cercariae of Schistosoma mansoni. J Parasitol. 1990 Jun;76(3):365–372. [PubMed] [Google Scholar]
- Kamijo R., Shapiro D., Le J., Huang S., Aguet M., Vilcek J. Generation of nitric oxide and induction of major histocompatibility complex class II antigen in macrophages from mice lacking the interferon gamma receptor. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6626–6630. doi: 10.1073/pnas.90.14.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E. A., Colley D. G. In vivo effects of monoclonal anti-L3T4 antibody on immune responsiveness of mice infected with Schistosoma mansoni. Reduction of irradiated cercariae-induced resistance. J Immunol. 1988 Apr 15;140(8):2737–2745. [PubMed] [Google Scholar]
- Lawson J. R., Wilson R. A. Metabolic changes associated with the migration of the schistosomulum of Schistosoma mansoni in the mammal host. Parasitology. 1980 Oct;81(2):325–336. doi: 10.1017/s0031182000056067. [DOI] [PubMed] [Google Scholar]
- Lazdins J. K., Stein M. J., David J. R., Sher A. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982 Feb;53(1):39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- Lewis F. A., White-Ziegler C. A., Ball J. E., Niemann G. M. Schistosoma mansoni larvicidal activity of murine bronchoalveolar lavage cells. Infect Immun. 1990 Dec;58(12):3903–3908. doi: 10.1128/iai.58.12.3903-3908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Moss D., Parkinson C., Rogers M. V., Moncada S. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991 Dec;21(12):3009–3014. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott N. A., Sutherland I. A., Sternberg J. M. Trypanosoma brucei is protected from the cytostatic effects of nitric oxide under in vivo conditions. Parasitol Res. 1994;80(8):687–690. doi: 10.1007/BF00932954. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Menson E. N., Coulson P. S., Wilson R. A. Schistosoma mansoni: circulating and pulmonary leucocyte responses related to the induction of protective immunity in mice by irradiated parasites. Parasitology. 1989 Feb;98(Pt 1):43–55. doi: 10.1017/s0031182000059679. [DOI] [PubMed] [Google Scholar]
- Oswald I. P., Eltoum I., Wynn T. A., Schwartz B., Caspar P., Paulin D., Sher A., James S. L. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):999–1003. doi: 10.1073/pnas.91.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., James S. L. Post lung-stage schistosomula of Schistosoma mansoni exhibit transient susceptibility to macrophage-mediated cytotoxicity in vitro that may relate to late phase killing in vivo. Parasite Immunol. 1986 Sep;8(5):513–527. doi: 10.1111/j.1365-3024.1986.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Pemberton R. M., Smythies L. E., Mountford A. P., Wilson R. A. Patterns of cytokine production and proliferation by T lymphocytes differ in mice vaccinated or infected with Schistosoma mansoni. Immunology. 1991 Jul;73(3):327–333. [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Gazzinelli G., Howells R. E., Mota-Santos T. A., Figueiredo E. A., Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp Parasitol. 1974 Dec;36(3):360–372. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Cheever A. W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990 Dec 1;145(11):3911–3916. [PubMed] [Google Scholar]
- Smythies L. E., Betts C., Coulson P. S., Dowling M. A., Wilson R. A. Kinetics and mechanism of effector focus formation in the lungs of mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasite Immunol. 1996 Jul;18(7):359–369. doi: 10.1046/j.1365-3024.1996.d01-115.x. [DOI] [PubMed] [Google Scholar]
- Smythies L. E., Coulson P. S., Wilson R. A. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992 Dec 1;149(11):3654–3658. [PubMed] [Google Scholar]
- Smythies L. E., Pemberton R. M., Coulson P. S., Mountford A. P., Wilson R. A. T cell-derived cytokines associated with pulmonary immune mechanisms in mice vaccinated with irradiated cercariae of Schistosoma mansoni. J Immunol. 1992 Mar 1;148(5):1512–1518. [PubMed] [Google Scholar]
- Sternberg J., Mabbott N., Sutherland I., Liew F. Y. Inhibition of nitric oxide synthesis leads to reduced parasitemia in murine Trypanosoma brucei infection. Infect Immun. 1994 May;62(5):2135–2137. doi: 10.1128/iai.62.5.2135-2137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali D. A., Crocker P., Bickle Q. D., Cobbold S., Waldmann H., Taylor M. G. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology. 1989 Aug;67(4):466–472. [PMC free article] [PubMed] [Google Scholar]
- Von Lichtenberg F., Correa-Oliveira R., Sher A. The fate of challenge schistosomula in the murine anti-schistosome vaccine model. Am J Trop Med Hyg. 1985 Jan;34(1):96–106. doi: 10.4269/ajtmh.1985.34.96. [DOI] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Wilson R. A., Coulson P. S., Betts C., Dowling M. A., Smythies L. E. Impaired immunity and altered pulmonary responses in mice with a disrupted interferon-gamma receptor gene exposed to the irradiated Schistosoma mansoni vaccine. Immunology. 1996 Feb;87(2):275–282. doi: 10.1046/j.1365-2567.1996.465550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. A., Coulson P. S. Schistosoma mansoni: dynamics of migration through the vascular system of the mouse. Parasitology. 1986 Feb;92(Pt 1):83–100. doi: 10.1017/s0031182000063472. [DOI] [PubMed] [Google Scholar]
- Wynn T. A., Oswald I. P., Eltoum I. A., Caspar P., Lowenstein C. J., Lewis F. A., James S. L., Sher A. Elevated expression of Th1 cytokines and nitric oxide synthase in the lungs of vaccinated mice after challenge infection with Schistosoma mansoni. J Immunol. 1994 Dec 1;153(11):5200–5209. [PubMed] [Google Scholar]


