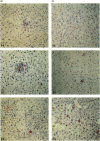
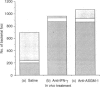
Abstract
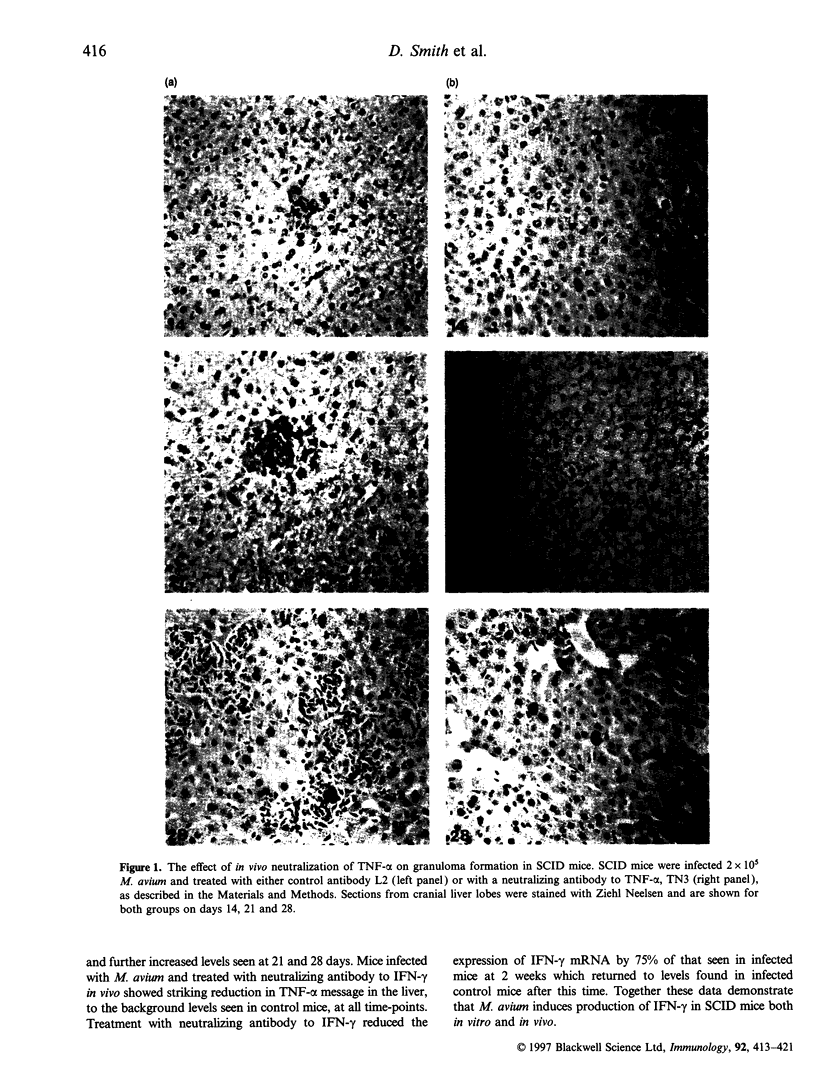
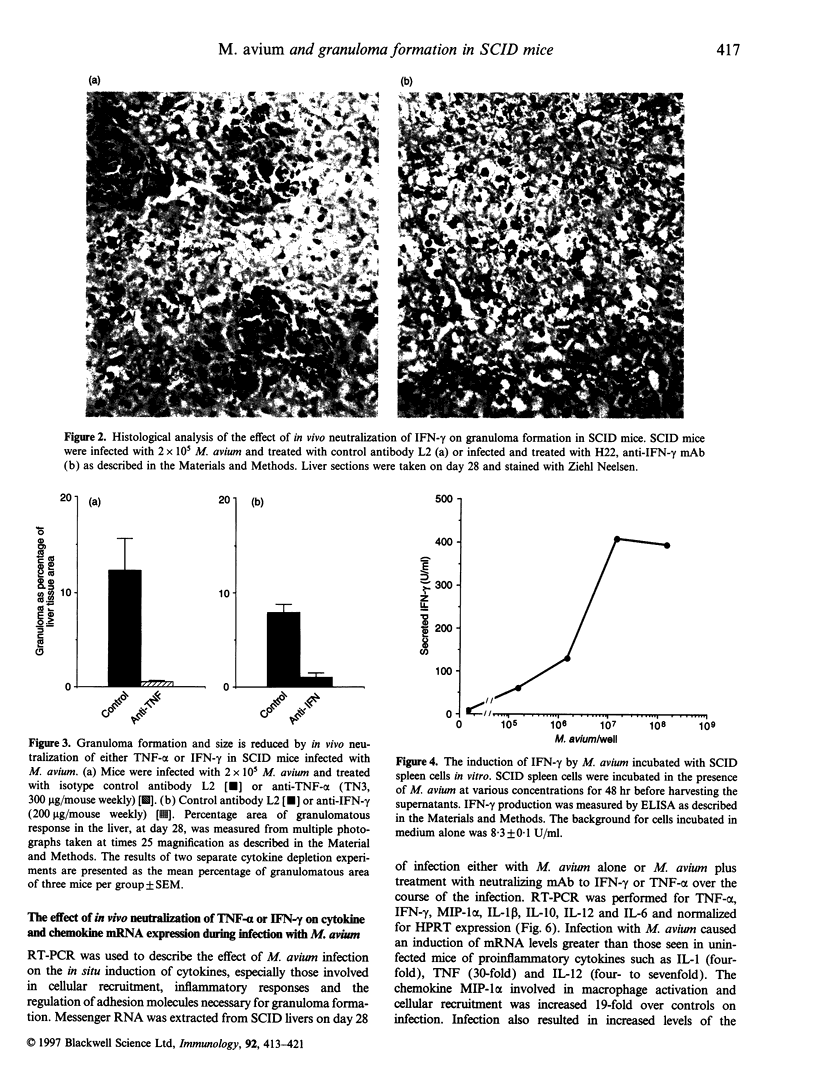
We used Mycobacterium avium infection in severe combined immunodeficiency (SCID) mice to examine T-cell-independent mechanisms of inflammatory cell recruitment. SCID mice infected with a virulent strain of M. avium (TMC724) were able to recruit macrophages to sites of mycobacterial replication and formed organized and coherent granulomas in the absence of functional T cells. Phagocyte recruitment was almost totally ablated by neutralization of either tumour necrosis factor-alpha (TNF-alpha) or interferon-gamma (IFN-gamma) in vivo demonstrating that granuloma formation was dependent on the presence of these cytokines. This was concomitant with a reduction in the in situ cytokine mRNA levels otherwise induced in infected mice, for chemokines, pro-inflammatory and regulatory cytokines, including TNF-alpha, IFN-gamma, macrophage inflammatory protein-1 alpha, interleukin-1 beta (IL-1 beta) and IL-10. Furthermore, in vivo treatment of infected mice with anti-asialo GM-1 antisera, which depletes natural killer (NK) cells, prevented recruitment of inflammatory cells. In vitro studies confirmed that M. avium was able to elicit IFN-gamma from SCID spleen in a dose-dependent manner. These data show for the first time that secretion of IFN-gamma from NK cells can mediate a T-cell-independent pathway of granuloma formation and cellular infiltration in response to mycobacteria.
Full text
PDF


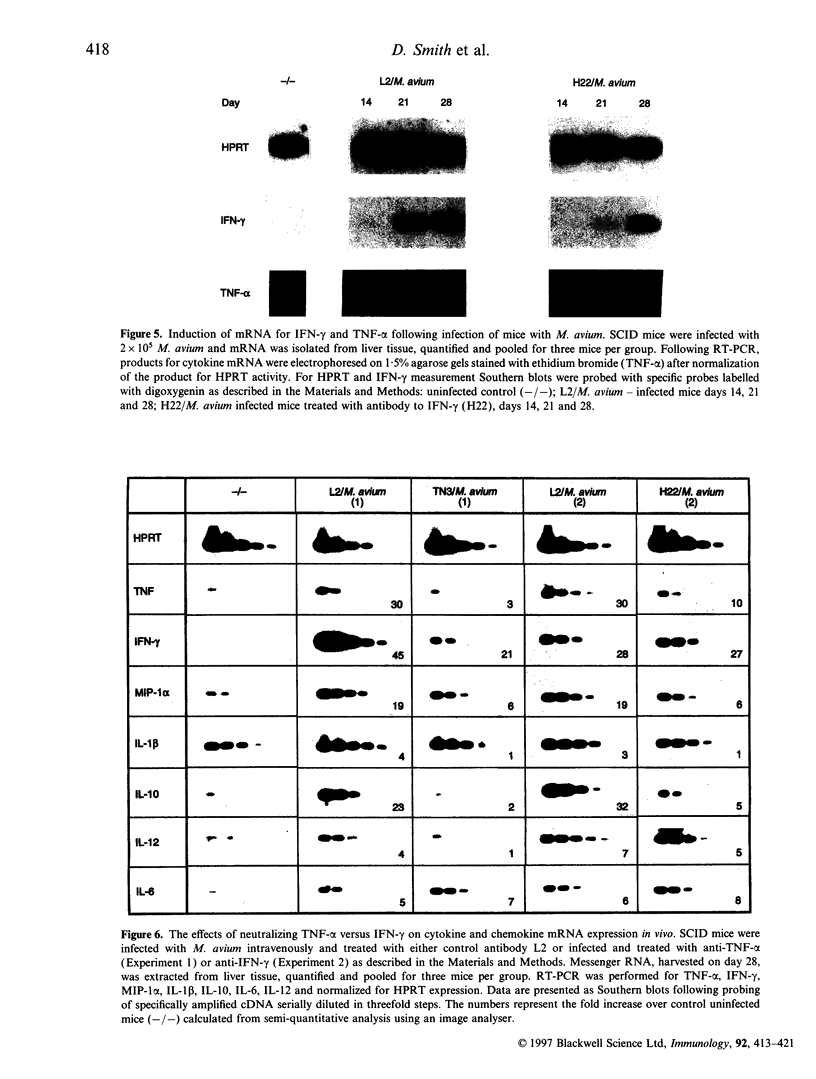
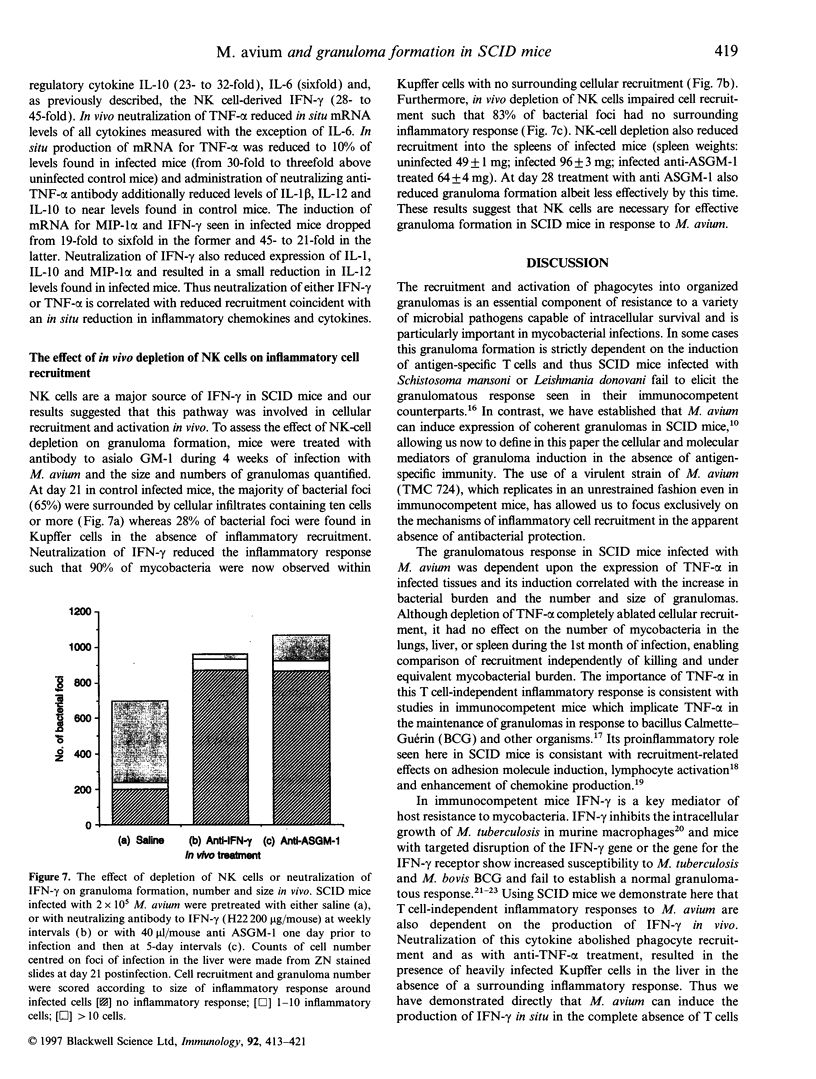


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam R., Forsythe P. A., Stafford S., Lett-Brown M. A., Grant J. A. Macrophage inflammatory protein-1 alpha activates basophils and mast cells. J Exp Med. 1992 Sep 1;176(3):781–786. doi: 10.1084/jem.176.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Castro A. G., Pedrosa J., Silva R. A., Orme I. M., Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994 Sep;62(9):3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R. Interferon-gamma (IFN-gamma) and macrophage inflammatory proteins (MIP)-1 and -2 are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin Exp Immunol. 1992 Aug;89(2):269–273. doi: 10.1111/j.1365-2249.1992.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Pedrosa J. Induction and expression of protective T cells during Mycobacterium avium infections in mice. Clin Exp Immunol. 1992 Mar;87(3):379–385. doi: 10.1111/j.1365-2249.1992.tb03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993 Aug;5(4):503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Kolonoski P., Young L. S. Natural killer cell activity and macrophage-dependent inhibition of growth or killing of Mycobacterium avium complex in a mouse model. J Leukoc Biol. 1990 Feb;47(2):135–141. doi: 10.1002/jlb.47.2.135. [DOI] [PubMed] [Google Scholar]
- Castro A. G., Silva R. A., Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995 Aug 15;155(4):2013–2019. [PubMed] [Google Scholar]
- Celada A., Klemsz M. J., Maki R. A. Interferon-gamma activates multiple pathways to regulate the expression of the genes for major histocompatibility class II I-A beta, tumor necrosis factor and complement component C3 in mouse macrophages. Eur J Immunol. 1989 Jun;19(6):1103–1109. doi: 10.1002/eji.1830190621. [DOI] [PubMed] [Google Scholar]
- Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993 Dec 1;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Bancroft G. J. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and beta-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect Immun. 1995 Jul;63(7):2604–2611. doi: 10.1128/iai.63.7.2604-2611.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatelis G., Tekamp-Olson P., Wolpe S. D., Hermsen K., Luedke C., Gallegos C., Coit D., Merryweather J., Cerami A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med. 1988 Jun 1;167(6):1939–1944. doi: 10.1084/jem.167.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Goldstein M. M., Chan J., Triebold K. J., Pfeffer K., Lowenstein C. J., Schreiber R., Mak T. W., Bloom B. R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995 Jun;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Fulton S. A., Johnsen J. M., Wolf S. F., Sieburth D. S., Boom W. H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996 Jul;64(7):2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshan K. V., Gangadharam P. R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991 Aug;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsch H. C., Smith D. A., Mielke M. E., Hahn H., Bancroft G. J., Ehlers S. Mechanisms of granuloma formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Int Immunol. 1996 Aug;8(8):1299–1310. doi: 10.1093/intimm/8.8.1299. [DOI] [PubMed] [Google Scholar]
- Kamijo R., Le J., Shapiro D., Havell E. A., Huang S., Aguet M., Bosland M., Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guérin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993 Oct 1;178(4):1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Bancroft G. J. Leishmania donovani infection in scid mice: lack of tissue response and in vivo macrophage activation correlates with failure to trigger natural killer cell-derived gamma interferon production in vitro. Infect Immun. 1992 Oct;60(10):4335–4342. doi: 10.1128/iai.60.10.4335-4342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. P., Bancroft G. J. Administration of interleukin-10 abolishes innate resistance to Listeria monocytogenes. Eur J Immunol. 1996 Feb;26(2):356–364. doi: 10.1002/eji.1830260214. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Kasama T., Yamazaki J., Hosaka M., Katsura T., Mochizuki T., Soejima K., Nakamura R. M. Protection of mice from Mycobacterium avium infection by recombinant interleukin-12. Antimicrob Agents Chemother. 1995 Jun;39(6):1369–1371. doi: 10.1128/aac.39.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel C. H., Hess J., Daugelat S., Mombaerts P., Tonegawa S., Kaufmann S. H. Contribution of alpha/beta and gamma/delta T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guérin: studies with T cell receptor-deficient mutant mice. Eur J Immunol. 1995 Mar;25(3):838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Zachariae C. O., Oppenheim J. J., Matsushima K. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1403–1408. doi: 10.1016/s0006-291x(89)80160-3. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Chensue S. W., Strieter R. M., Warmington K., Kunkel S. L. Inflammatory granuloma formation is mediated by TNF-alpha-inducible intercellular adhesion molecule-1. J Immunol. 1994 Jun 15;152(12):5883–5889. [PubMed] [Google Scholar]
- Murphy E., Hieny S., Sher A., O'Garra A. Detection of in vivo expression of interleukin-10 using a semi-quantitative polymerase chain reaction method in Schistosoma mansoni infected mice. J Immunol Methods. 1993 Jun 18;162(2):211–223. doi: 10.1016/0022-1759(93)90386-l. [DOI] [PubMed] [Google Scholar]
- North R. J., Izzo A. A. Granuloma formation in severe combined immunodeficient (SCID) mice in response to progressive BCG infection. Tendency not to form granulomas in the lung is associated with faster bacterial growth in this organ. Am J Pathol. 1993 Jun;142(6):1959–1966. [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Furney S. K., Roberts A. D. Dissemination of enteric Mycobacterium avium infections in mice rendered immunodeficient by thymectomy and CD4 depletion or by prior infection with murine AIDS retroviruses. Infect Immun. 1992 Nov;60(11):4747–4753. doi: 10.1128/iai.60.11.4747-4753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Zheng S., Wang Z. E., Stowring L., Locksley R. M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994 Feb 1;179(2):447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades E. R., Cooper A. M., Orme I. M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995 Oct;63(10):3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Oswald I. P., Hieny S., Gazzinelli R. T. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol. 1993 May 1;150(9):3982–3989. [PubMed] [Google Scholar]
- Taub D. D., Conlon K., Lloyd A. R., Oppenheim J. J., Kelvin D. J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993 Apr 16;260(5106):355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]