Abstract
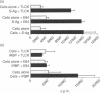
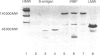
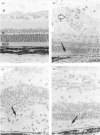
We have found that different antigen-processing pathways are involved in the induction of experimental autoimmune uveoretinitis (EAU) by the retinal autoantigens S-antigen and interphotoreceptor retinoid-binding protein (IRBP). Although in vitro T-cell proliferative responses to IRBP were completely inhibited in the presence of irreversible cysteine protease inhibitors, no significant reduction of S-antigen proliferative responses was found. Furthermore, acidic proteolysis of S-antigen by the cysteine protease cathepsin B prior to immunization radically reduced pathogenicity (disease severity). In addition, in vitro processing of S-antigen, but not IRBP, was also found to be resistant to the action of cycloheximide and lysosomotropic agents, inhibition of proliferation only occurring after extended exposure of antigen-presenting cells to methyl amine or high concentrations of chloroquine. These data indicate that an alternative pathway of antigen processing exists for S-antigen, which is independent of processing within the normal endolysosomal pathway and that uveitogenic peptides of naturally processed S-antigen bind to major histocompatibility complex class II antigens either at the cell surface or within very early endosomes where cathepsin B is inactive.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Guéry J. C. Suppression of T-cell activation by administration of MHC class II-binding peptides. Immunol Ser. 1993;59:331–343. [PubMed] [Google Scholar]
- Amigorena S., Drake J. R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994 May 12;369(6476):113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Anderson M. S., Swier K., Arneson L., Miller J. Enhanced antigen presentation in the absence of the invariant chain endosomal localization signal. J Exp Med. 1993 Dec 1;178(6):1959–1969. doi: 10.1084/jem.178.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chader G. J. Interphotoreceptor retinoid-binding protein (IRBP): a model protein for molecular biological and clinically relevant studies. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):7–22. [PubMed] [Google Scholar]
- Collins D. S., Unanue E. R., Harding C. V. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991 Dec 15;147(12):4054–4059. [PubMed] [Google Scholar]
- Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996 Feb 23;84(4):505–507. doi: 10.1016/s0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Denzin L. K., Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995 Jul 14;82(1):155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., Liversidge J., Forrester J. V. Immunomodulation of experimental autoimmune uveoretinitis: a model of tolerance induction with retinal antigens. Eye (Lond) 1994;8(Pt 1):52–59. doi: 10.1038/eye.1994.10. [DOI] [PubMed] [Google Scholar]
- Donoso L. A., Yamaki K., Merryman C. F., Shinohara T., Yue S., Sery T. W. Human S-antigen: characterization of uveitopathogenic sites. Curr Eye Res. 1988 Nov;7(11):1077–1085. doi: 10.3109/02713688809001878. [DOI] [PubMed] [Google Scholar]
- Forrester J. V. Uveitis: pathogenesis. Lancet. 1991 Dec 14;338(8781):1498–1501. doi: 10.1016/0140-6736(91)92309-p. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Fling S. P., Obritsch W. F., Merryman C. F., Donoso L. A. Identification of T cell recognition sites in S-antigen: dissociation of proliferative and pathogenic sites. Cell Immunol. 1989 Oct 15;123(2):427–440. doi: 10.1016/0008-8749(89)90302-x. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990 Aug 9;346(6284):574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- Harper F. H., Liversidge J., Thomson A. W., Forrester J. V. Interphotoreceptor retinoid binding protein induced experimental autoimmune uveitis: an immunophenotypic analysis using alkaline phosphatase anti-alkaline phosphatase staining, dual immunofluorescence and confocal microscopy. Curr Eye Res. 1992;11 (Suppl):129–134. doi: 10.3109/02713689208999522. [DOI] [PubMed] [Google Scholar]
- Isaacs J., Dick A. Short term immunosuppressive therapy and long-term immunoregulation: promises and problems. Br J Ophthalmol. 1996 Dec;80(12):1035–1036. doi: 10.1136/bjo.80.12.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhich A. T., Kawano Y., Egwuagu C. E., Caspi R. R., Maturi R. K., Berzofsky J. A., Gery I. A pathogenic autoimmune process targeted at a surrogate epitope. J Exp Med. 1994 Jul 1;180(1):133–140. doi: 10.1084/jem.180.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Watts C. Peptide partners call the tune. Nature. 1994 Sep 15;371(6494):198–199. doi: 10.1038/371198a0. [DOI] [PubMed] [Google Scholar]
- Liversidge J., Forrester J. V. Antigen processing and presentation in the eye: a review. Curr Eye Res. 1992;11 (Suppl):49–58. doi: 10.3109/02713689208999511. [DOI] [PubMed] [Google Scholar]
- Liversidge J., Thomson A. W., Sewell H. F., Forrester J. V. EAU in the guinea pig: inhibition of cell-mediated immunity and Ia antigen expression by cyclosporin A. Clin Exp Immunol. 1987 Sep;69(3):591–600. [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y., Saibara T., Kido H., Katunuma N. Participation of cathepsin B in processing of antigen presentation to MHC class II. FEBS Lett. 1993 Jun 21;324(3):325–330. doi: 10.1016/0014-5793(93)80144-j. [DOI] [PubMed] [Google Scholar]
- Pinet V., Vergelli M., Martin R., Bakke O., Long E. O. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995 Jun 15;375(6532):603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- Polgár L., Csoma C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. J Biol Chem. 1987 Oct 25;262(30):14448–14453. [PubMed] [Google Scholar]
- Riese R. J., Wolf P. R., Brömme D., Natkin L. R., Villadangos J. A., Ploegh H. L., Chapman H. A. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996 Apr;4(4):357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- Santoro L., Reboul A., Journet A. M., Colomb M. G. Major involvement of cathepsin B in the intracellular proteolytic processing of exogenous IgGs in U937 cells. Mol Immunol. 1993 Aug;30(11):1033–1039. doi: 10.1016/0161-5890(93)90128-x. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Jackson M. R. Immunology. Making class II presentable. Nature. 1994 May 12;369(6476):103–104. doi: 10.1038/369103a0. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E., Lehmann P. V., Ametani A., Benichou G., Miller A., Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- Singh D. P., Kikuchi T., Singh V. K., Shinohara T. A single amino acid substitution in core residues of S-antigen prevents experimental autoimmune uveitis. J Immunol. 1994 May 1;152(9):4699–4705. [PubMed] [Google Scholar]
- Vergelli M., Pinet V., Vogt A. B., Kalbus M., Malnati M., Riccio P., Long E. O., Martin R. HLA-DR-restricted presentation of purified myelin basic protein is independent of intracellular processing. Eur J Immunol. 1997 Apr;27(4):941–951. doi: 10.1002/eji.1830270421. [DOI] [PubMed] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- Wolf P. R., Ploegh H. L. Antigen presentation. DM exchange mechanism. Nature. 1995 Aug 10;376(6540):464–465. doi: 10.1038/376464a0. [DOI] [PubMed] [Google Scholar]