Abstract
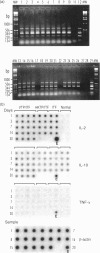
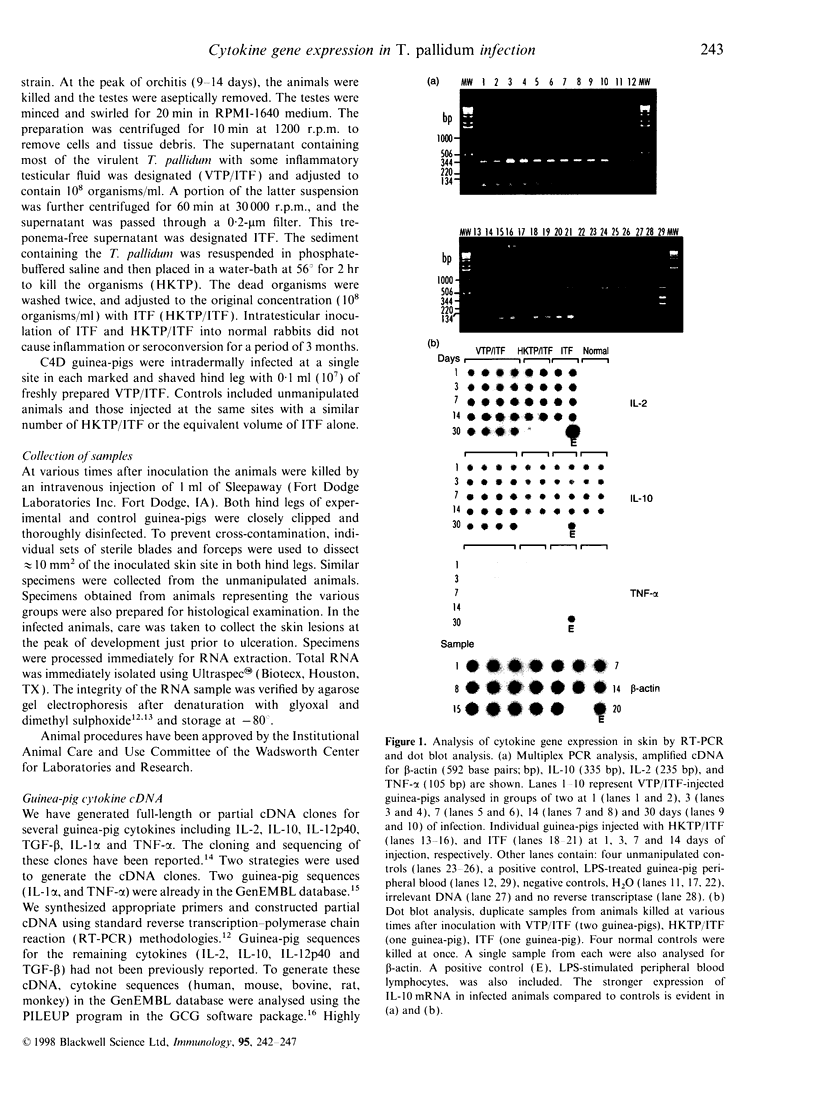
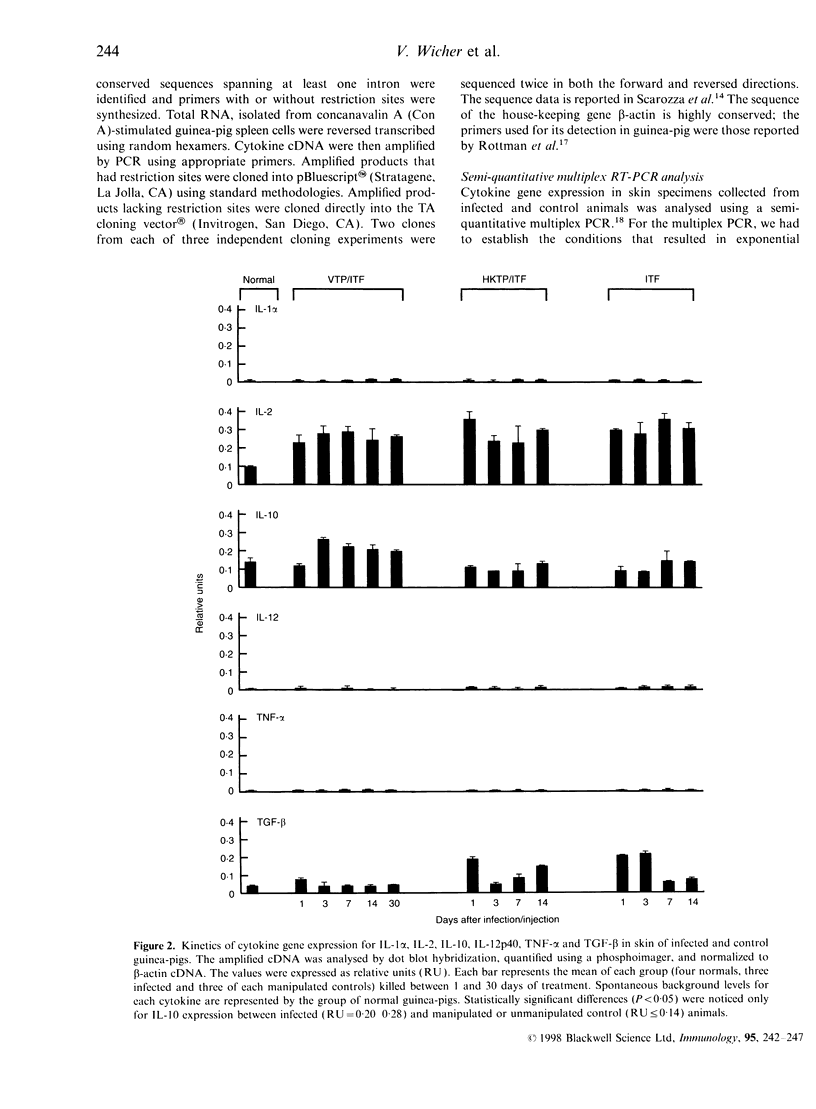
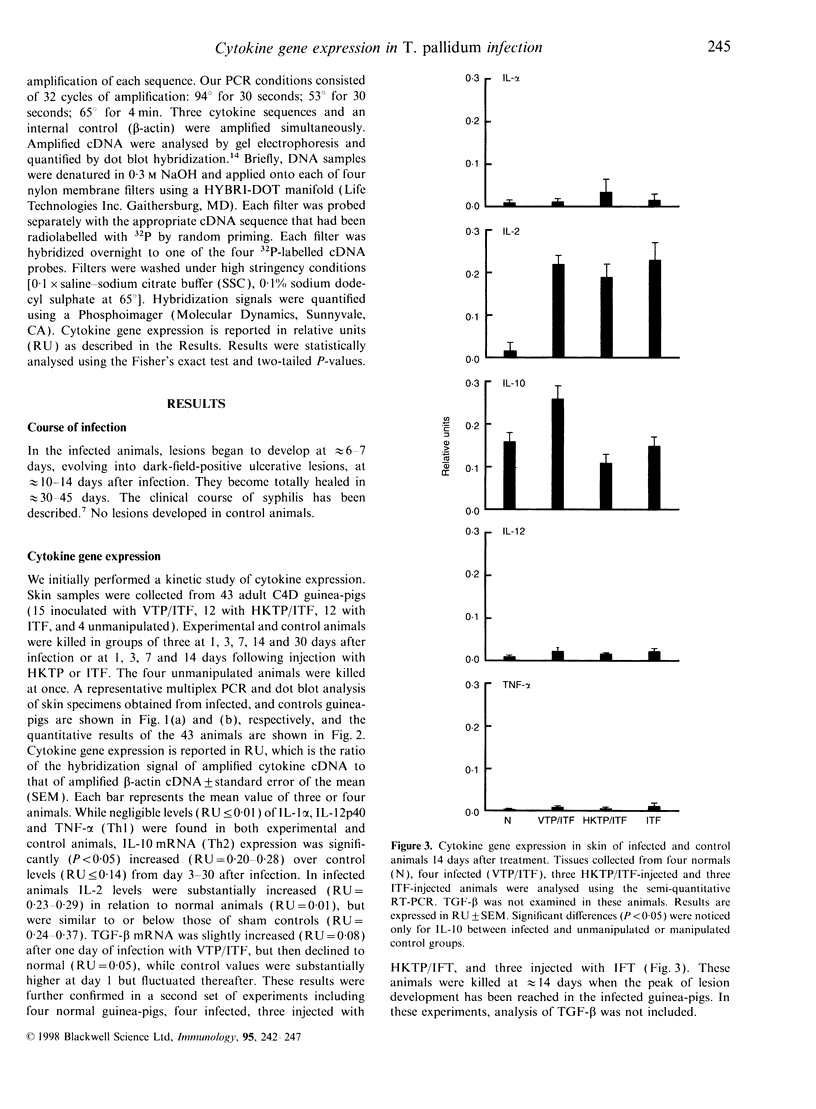
Using a semi-quantitative multiplex reverse transcription-polymerase chain reaction assay, we examined cytokine mRNA expression for interleukin-1alpha (IL-1alpha), IL-2, IL-10, IL-12p40, tumour necrosis factor-alpha (TNF-alpha) and transforming growth factor-beta (TGF-beta) in skin samples obtained from C4-deficient (C4D) guinea-pigs inoculated intradermally with virulent Treponema pallidum (VTP). Controls included unmanipulated animals, guinea-pigs injected with T. pallidum-free rabbit inflammatory testicular fluid (ITF) alone, or mixed with heat-killed organisms (HKTP). The expression of IL-1alpha, IL-12p40, and TNF-alpha mRNA [T helper type 1 (Th1)] remained within the normal range in both infected and control animals throughout the experimental period. However, a significant increase (P<0.05) in IL-10 mRNA (Th2) was found exclusively in the VTP-inoculated animals from 3 to 30 days post-infection. Another unique characteristic of the inflammatory response in infected guinea-pigs was the appearance, between 11 and 30 days post-inoculation, of a substantial number of eosinophils in addition to infiltrating mononuclear cells. The results showed a local Th2 response which is consistent with an inadequate immune response. This is reflected by the lengthy and incomplete clearance of the pathogen from the local site of entry and the chronic infection of distant organs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R., Shevach E. M. Evaluation of the role of C4 in the cellular immune response in vitro. J Immunol. 1979 Jun;122(6):2388–2394. [PubMed] [Google Scholar]
- Ellman L., Green I., Frank M. Genetically controlled total deficiency of the fourth component of complement in the guinea pig. Science. 1970 Oct 2;170(3953):74–75. doi: 10.1126/science.170.3953.74. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J. The Th1/Th2-like switch in syphilitic infection: is it detrimental? Infect Immun. 1992 Sep;60(9):3475–3479. doi: 10.1128/iai.60.9.3475-3479.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., May J., Gaither T., Ellman L. In vitro studies of complement function in sera of C4-deficient guinea pigs. J Exp Med. 1971 Jul 1;134(1):176–187. doi: 10.1084/jem.134.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. L., Scales R. W., Kraus S. J. Increased serum immunoglobulin E concentrations in venereal diseases. Br J Vener Dis. 1976 Aug;52(4):257–260. doi: 10.1136/sti.52.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P., Delneste Y., Seveso M., Life P., Bonnefoy J. Y. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J Immunol. 1996 May 1;156(9):3159–3165. [PubMed] [Google Scholar]
- Lamont A. G., Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996 May;17(5):214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- Meyaard L., Hovenkamp E., Otto S. A., Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996 Apr 15;156(8):2776–2782. [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Spaccapelo R., Mosci P., Puccetti P., Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993 Feb 1;150(3):925–931. [PubMed] [Google Scholar]
- Rottman J. B., Freeman E. B., Tonkonogy S., Tompkins M. B. A reverse transcription-polymerase chain reaction technique to detect feline cytokine genes. Vet Immunol Immunopathol. 1995 Mar;45(1-2):1–18. doi: 10.1016/0165-2427(94)05324-l. [DOI] [PubMed] [Google Scholar]
- Silva J. S., Morrissey P. J., Grabstein K. H., Mohler K. M., Anderson D., Reed S. G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992 Jan 1;175(1):169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M. CD4+ T cell subsets. Lymphokine secretion of memory cells and of effector cells that develop from precursors in vitro. J Immunol. 1990 Mar 1;144(5):1788–1799. [PubMed] [Google Scholar]
- Van Voorhis W. C., Barrett L. K., Koelle D. M., Nasio J. M., Plummer F. A., Lukehart S. A. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J Infect Dis. 1996 Feb;173(2):491–495. doi: 10.1093/infdis/173.2.491. [DOI] [PubMed] [Google Scholar]
- Weller P. F. Eosinophils: structure and functions. Curr Opin Immunol. 1994 Feb;6(1):85–90. doi: 10.1016/0952-7915(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Wicher K., Abbruscato F., Wicher V., Baughn R., Noordhoek G. T. Target organs of infection in guinea pigs with acquired congenital syphilis. Infect Immun. 1996 Aug;64(8):3174–3179. doi: 10.1128/iai.64.8.3174-3179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher K., Abbruscato F., Wicher V., Collins D. N., Auger I., Horowitz H. W. Identification of persistent infection in experimental syphilis by PCR. Infect Immun. 1998 Jun;66(6):2509–2513. doi: 10.1128/iai.66.6.2509-2513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher K., Wicher V. Experimental syphilis in guinea pig. Crit Rev Microbiol. 1989;16(3):181–234. doi: 10.3109/10408418909104471. [DOI] [PubMed] [Google Scholar]
- Wicher K., Wicher V., Gruhn R. F. Differences in susceptibility to infection with Treponema pallidum (Nichols) between five strains of guinea pig. Genitourin Med. 1985 Feb;61(1):21–26. doi: 10.1136/sti.61.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K., Jakubowski A., Nakeeb S. M. Adoptive transfer of immunity to Treponema pallidum Nichols infection in inbred strain 2 and C4D guinea pigs. Infect Immun. 1987 Oct;55(10):2502–2508. doi: 10.1128/iai.55.10.2502-2508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Zabek J., Wicher K. Pathogen-specific humoral response in Treponema pallidum-infected humans, rabbits, and guinea pigs. J Infect Dis. 1991 Apr;163(4):830–836. [PubMed] [Google Scholar]
- Yobs A. R., Clark J. W., Jr, Mothershed S. E., Bullard J. C., Artley C. W. Further observations on the persistence of Treponema pallidum after treatment in rabbits and humans. Br J Vener Dis. 1968 Jun;44(2):116–130. doi: 10.1136/sti.44.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogeswari L., Chacko C. W. Persistence of T. pallidum and its significance in penicillin-treated seropositive late syphilis. Br J Vener Dis. 1971 Oct;47(5):339–347. doi: 10.1136/sti.47.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]