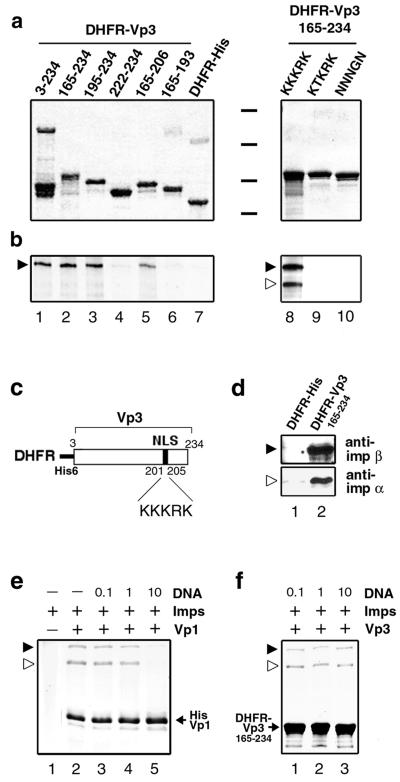
FIG. 5.
In vitro binding of Vps with importins α2 and β. (a and b) An equimolar amount of DHFR-Vp3 fusion proteins immobilized onto beads was individually incubated with either a mixture of cold GST-importin α and 125I-labeled GST-importin β (lanes 1 through 7) or 125I-labeled GST-importin α and 125I-labeled GST-importin β (lanes 8 through 10). The bead-bound proteins were analyzed by SDS-PAGE followed by Coomassie blue staining (a) and autoradiography (b). Black bars between the two subpanels in panel a indicate positions for molecular mass markers of 97.4, 66, 46, and 30 kDa. Locations for GST-importin α (open arrowhead) and GST-importin β (filled arrowhead) are marked. (c) Schematic diagram of recombinant DHFR-Vp33-234 protein. (d) DHFR-Vp3 can recognize endogenous importin α/β. The beads bound with either DHFR-His (lane 1) or DHFR-Vp3165-234 (lane 2) were mixed with the uninfected TC-7 cell lysate. The cellular proteins bound to the beads were probed with either anti-importin α (anti-imp α) or anti-importin β (anti-imp β) antibody by Western blotting. (e) The presence of DNA blocks importin α/β-Vp1 interaction. GST-importin α/β (Imps) was incubated either with or without histidine-tagged Vp1ΔC58 (Vp1) in the presence or absence of the DNA fragment (DNA). The molecular ratio of DNA to importins in each reaction mixture is marked on the respective lane. The Vp1ΔC58-importin complex was recovered with Talon beads and resolved by SDS-PAGE, and the bead-bound proteins were visualized by Coomassie blue staining. (f) Importin α/β binding with Vp3 is not blocked by DNA. Interaction of GST-importin α and β and bead-bound DHFR-VP3165-234 in the presence of DNA was examined as for panel d.