Abstract
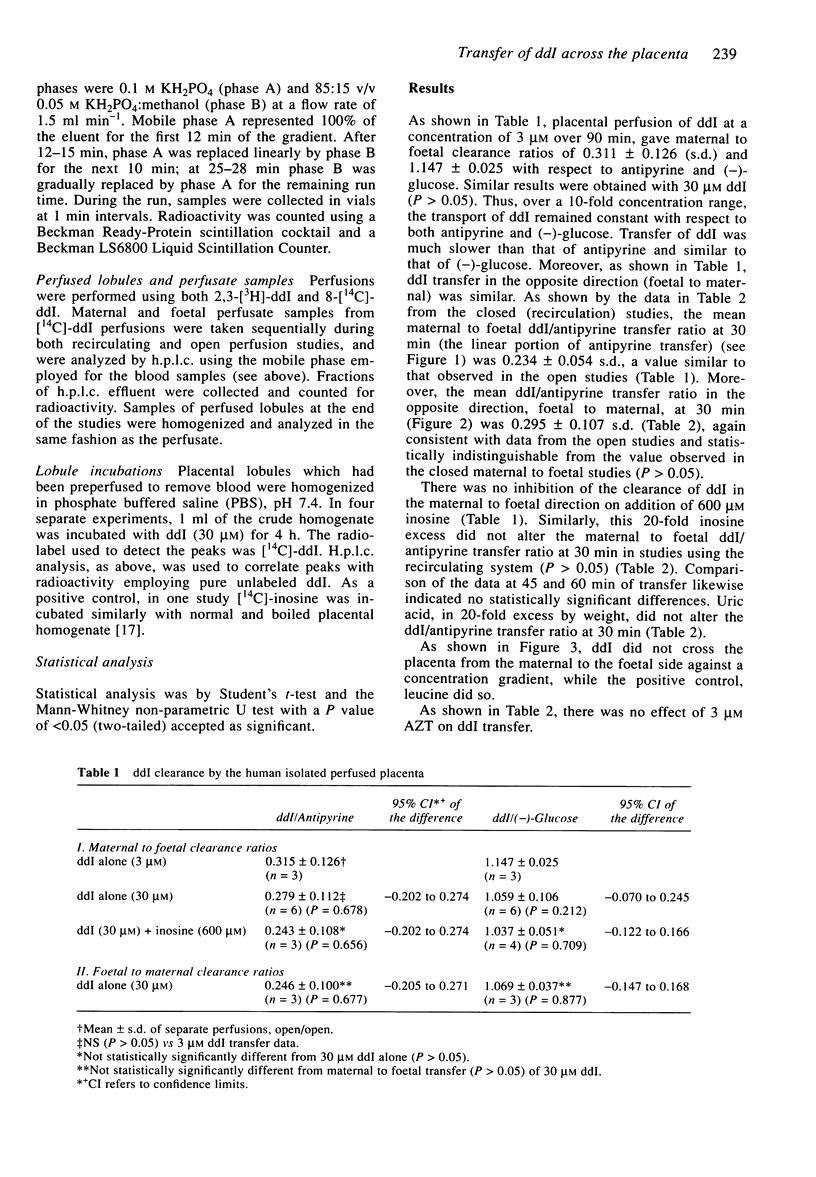
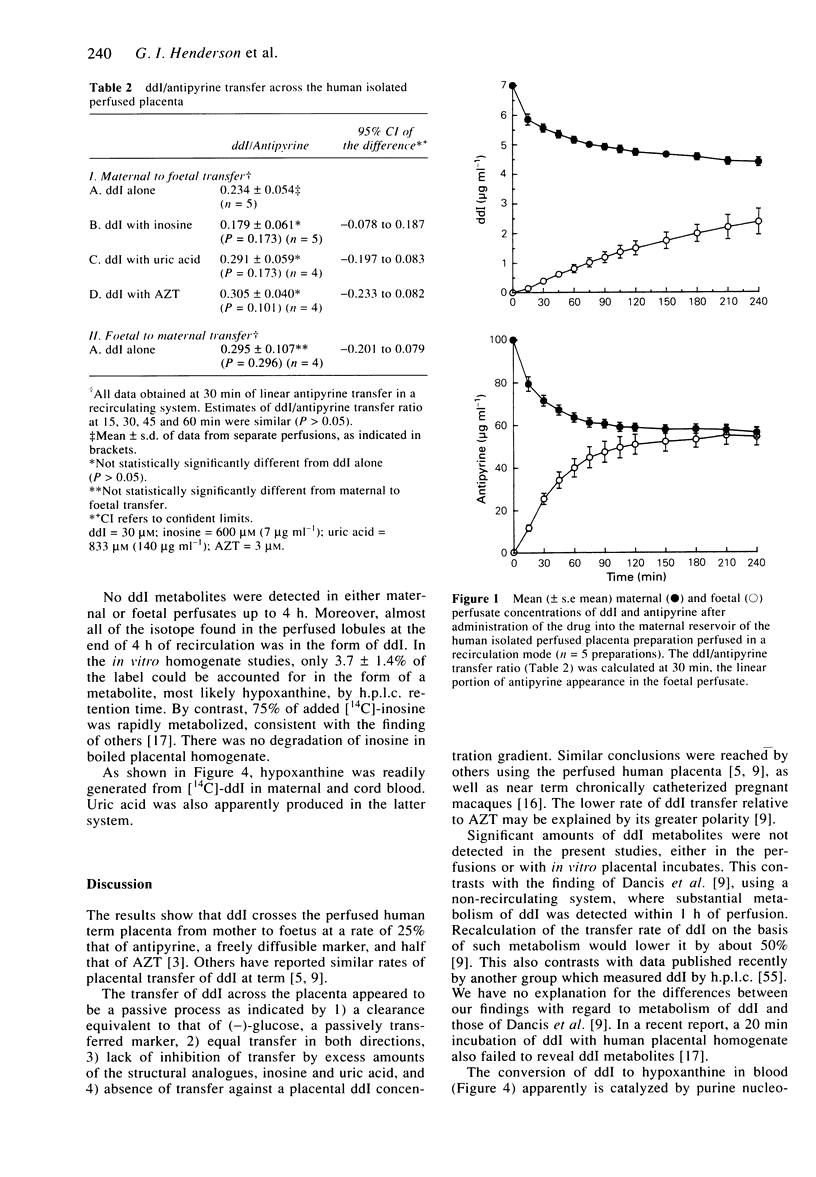
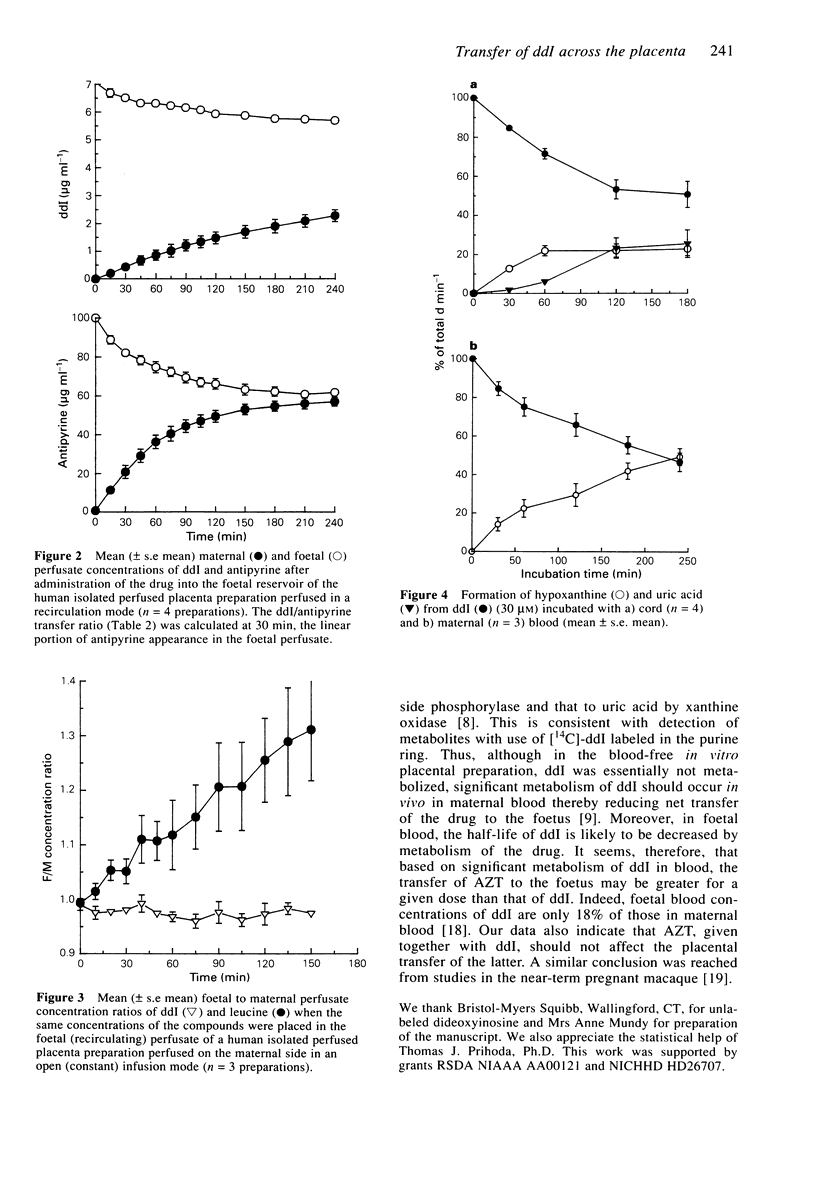
1. Dideoxyinosine (ddI) has recently been approved for the treatment of patients with HIV infection. As increasing numbers of such patients are pregnant, we wished to define the rate and mechanism(s) of ddI transfer by the placenta to the foetus. Using isolated single perfused human term placental cotyledons, the drug was shown to cross the placenta from mother to foetus at a rate of 25% that of a freely diffusible marker, antipyrine, and at about half the rate of zidovudine (AZT). The transfer of ddI was similar in both directions (maternal to foetal and the reverse), equal to that of L-glucose, a passively transported sugar, and was not inhibited by excess inosine or uric acid (structural analogues of ddI). ddI did not cross to the foetus against a concentration gradient. The transport process appeared to be passive and it was not altered by AZT. 2. ddI was not metabolized in the Krebs Ringer buffer/albumin perfusate, and placental homogenates converted only 4% of ddI to hypoxanthine over the 4 h incubation. However, when maternal term or cord blood was incubated with ddI for 3 h, 50% of the drug was converted to hypoxanthine in maternal blood and to hypoxanthine and uric acid in cord blood. 3. Thus, ddI metabolism in maternal blood should decrease its net transfer to the foetus in vivo. In the foetal circulation, ddI will be further metabolized by erythrocytes to hypoxanthine and possibly to uric acid. Hence, the fraction of administered ddI delivered to foetal tissues should be much lower than that of AZT.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. J., Ormesher S., Tjia J. F., Macleod R. Metabolism of 2',3'-dideoxyinosine (ddI) in human blood. Br J Clin Pharmacol. 1992 Mar;33(3):319–322. doi: 10.1111/j.1365-2125.1992.tb04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawdon R. E., Sobhi S., Dax J. The transfer of anti-human immunodeficiency virus nucleoside compounds by the term human placenta. Am J Obstet Gynecol. 1992 Dec;167(6):1570–1574. doi: 10.1016/0002-9378(92)91742-s. [DOI] [PubMed] [Google Scholar]
- Brandes J. M., Tavoloni N., Potter B. J., Sarkozi L., Shepard M. D., Berk P. D. A new recycling technique for human placental cotyledon perfusion: application to studies of the fetomaternal transfer of glucose, inulin, and antipyrine. Am J Obstet Gynecol. 1983 Aug 1;146(7):800–806. doi: 10.1016/0002-9378(83)91081-5. [DOI] [PubMed] [Google Scholar]
- Dalton J. T., Au J. L. 2',3'-Dideoxyinosine is not metabolized in human placenta. Drug Metab Dispos. 1993 May-Jun;21(3):544–546. [PubMed] [Google Scholar]
- Hartman N. R., Yarchoan R., Pluda J. M., Thomas R. V., Marczyk K. S., Broder S., Johns D. G. Pharmacokinetics of 2',3'-dideoxyadenosine and 2',3'-dideoxyinosine in patients with severe human immunodeficiency virus infection. Clin Pharmacol Ther. 1990 May;47(5):647–654. doi: 10.1038/clpt.1990.86. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., D'Aquila R. T. Therapy for human immunodeficiency virus infection. N Engl J Med. 1993 Jun 10;328(23):1686–1695. doi: 10.1056/NEJM199306103282307. [DOI] [PubMed] [Google Scholar]
- Liebes L., Mendoza S., Wilson D., Dancis J. Transfer of zidovudine (AZT) by human placenta. J Infect Dis. 1990 Feb;161(2):203–207. doi: 10.1093/infdis/161.2.203. [DOI] [PubMed] [Google Scholar]
- Lockwood G. F., Houston J. B. Amidopyrine disposition in rat. J Pharm Pharmacol. 1979 Nov;31(11):787–788. doi: 10.1111/j.2042-7158.1979.tb13661.x. [DOI] [PubMed] [Google Scholar]
- Pons J. C., Boubon M. C., Taburet A. M., Singlas E., Chambrin V., Frydman R., Papiernik E., Delfraissy J. F. Fetoplacental passage of 2',3'-dideoxyinosine. Lancet. 1991 Mar 23;337(8743):732–732. doi: 10.1016/0140-6736(91)90313-e. [DOI] [PubMed] [Google Scholar]
- Schenker S., Dicke J., Johnson R. F., Mor L. L., Henderson G. I. Human placental transport of cimetidine. J Clin Invest. 1987 Nov;80(5):1428–1434. doi: 10.1172/JCI113222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker S., Johnson R. F., Hoyumpa A. M., Henderson G. I. Thiamine-transfer by human placenta: normal transport and effects of ethanol. J Lab Clin Med. 1990 Jul;116(1):106–115. [PubMed] [Google Scholar]
- Schenker S., Johnson R. F., King T. S., Schenken R. S., Henderson G. I. Azidothymidine (zidovudine) transport by the human placenta. Am J Med Sci. 1990 Jan;299(1):16–20. doi: 10.1097/00000441-199001000-00005. [DOI] [PubMed] [Google Scholar]
- Schenker S., Johnson R. F., Mahuren J. D., Henderson G. I., Coburn S. P. Human placental vitamin B6 (pyridoxal) transport: normal characteristics and effects of ethanol. Am J Physiol. 1992 Jun;262(6 Pt 2):R966–R974. doi: 10.1152/ajpregu.1992.262.6.R966. [DOI] [PubMed] [Google Scholar]
- Schneider H., Panigel M., Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol. 1972 Nov 15;114(6):822–828. doi: 10.1016/0002-9378(72)90909-x. [DOI] [PubMed] [Google Scholar]


