Abstract
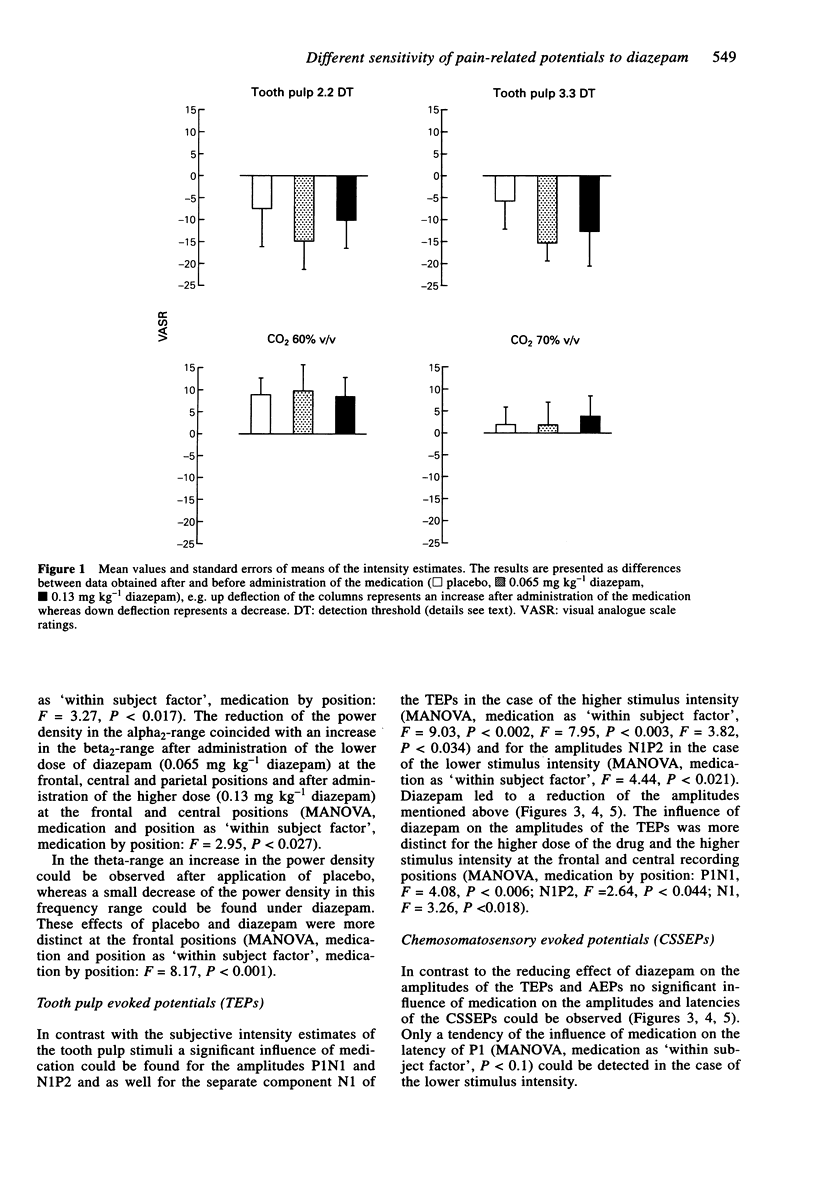
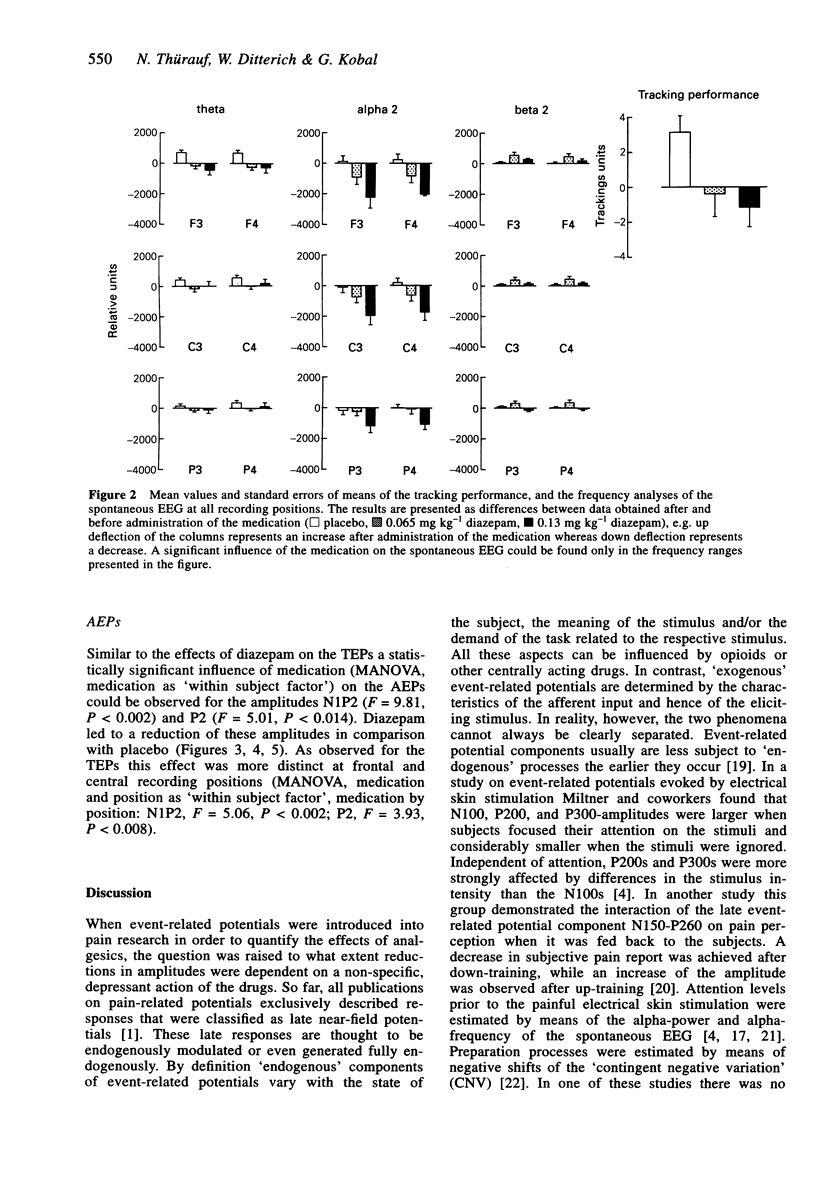
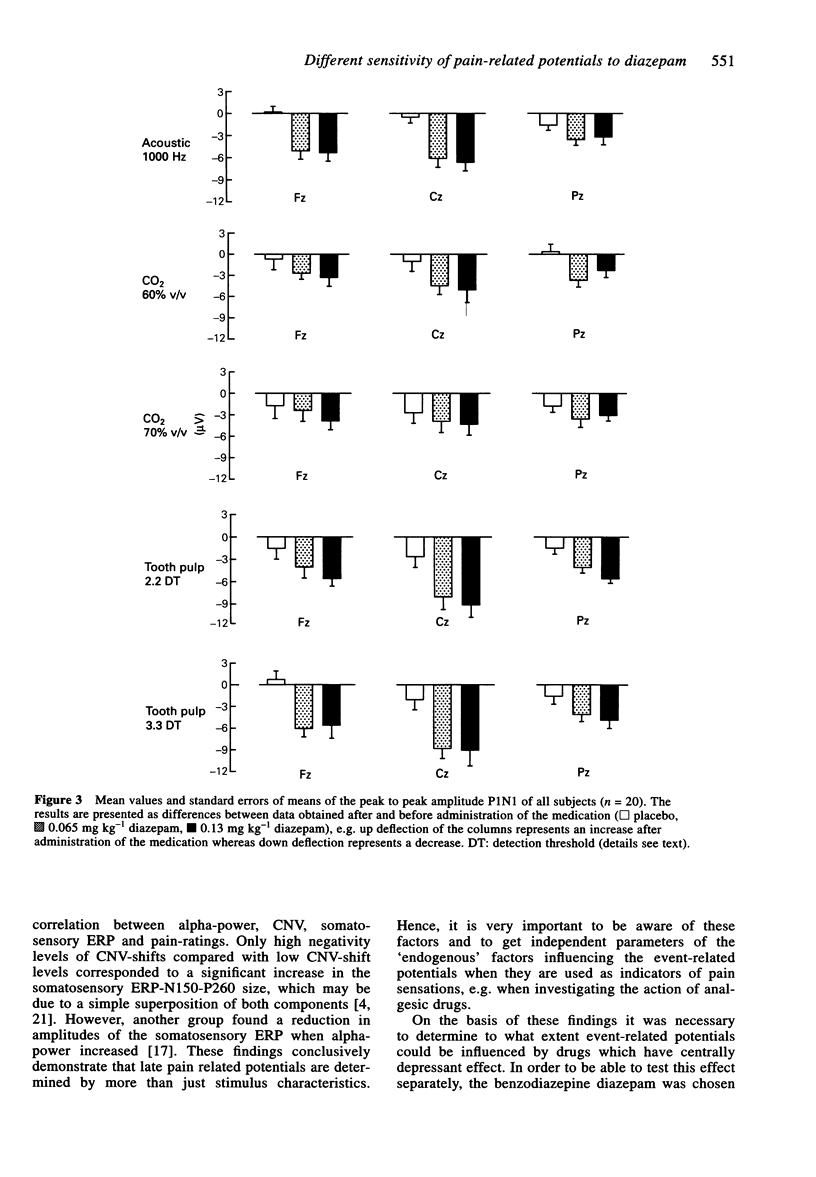
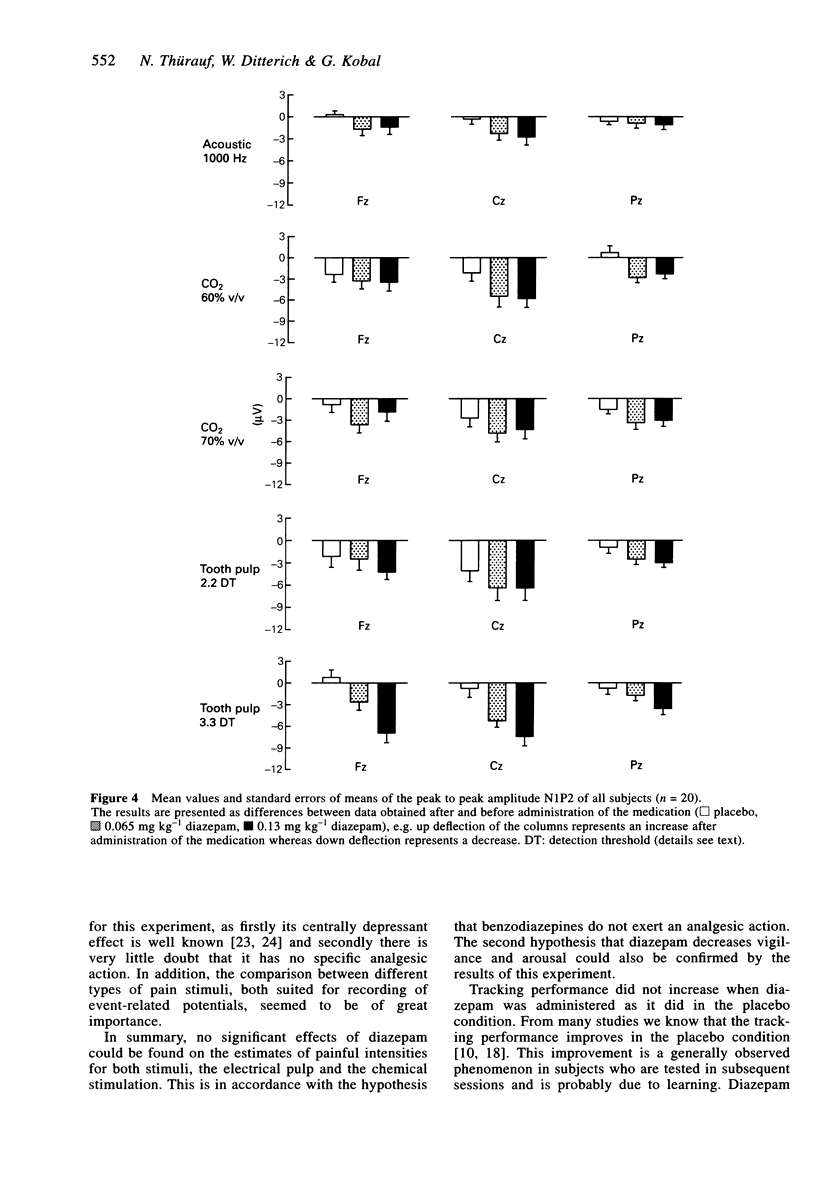
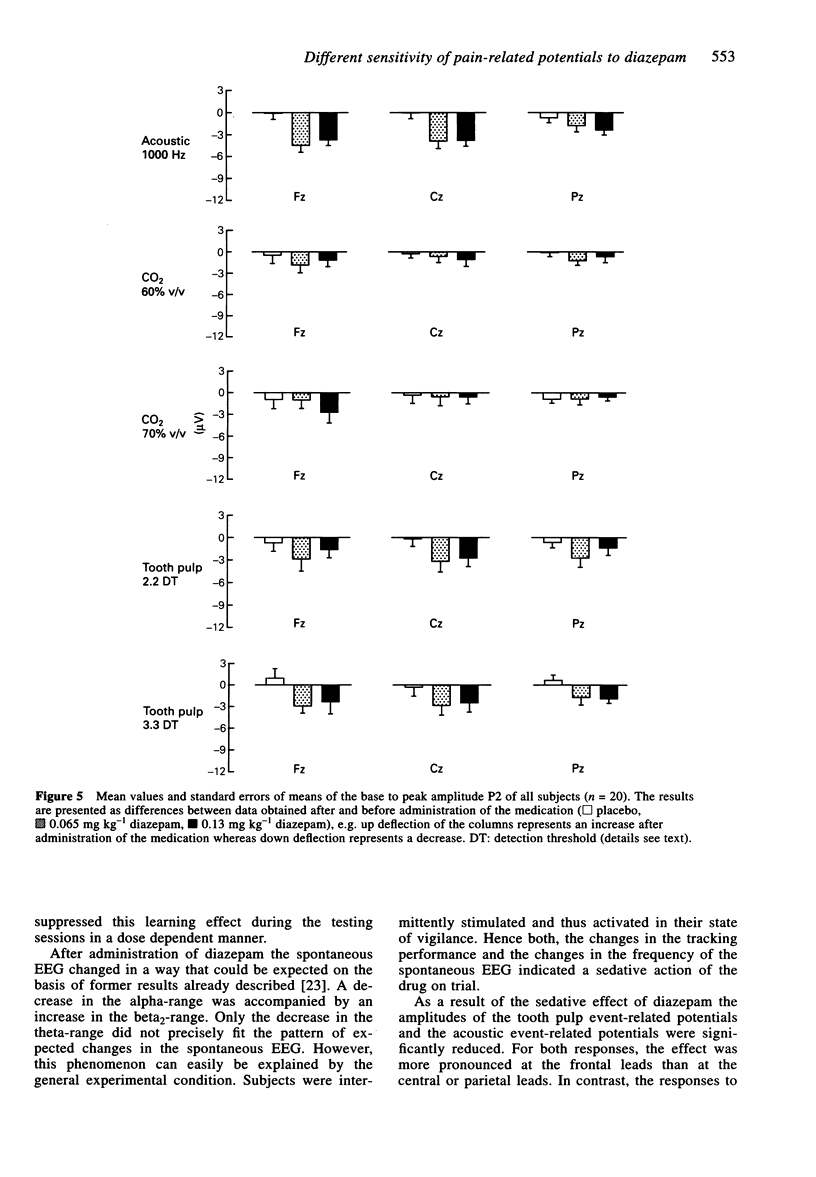
1. The aim of this study was to investigate the sensitivity of pain-related potentials used in experimental pain models to the non-specific effects of the tranquilizer diazepam. Pain-related potentials were recorded after painful stimulation of the nasal mucosa with CO2 and after painful stimulation of the tooth pulp. Acoustically evoked potentials were measured in order to compare their sensitivity to the tranquilizer diazepam with the sensitivity of the pain-related potentials. 2. Twenty volunteers participated in this randomised, double-blind, three-fold crossover study. Measurements were obtained before and 20 min after the administration of the drug. Event-related potentials were recorded after painful stimulation of the nasal mucosa with CO2 (two stimulus intensities: 60% v/v and 70% v/v CO2), after painful stimulation of the tooth pulp (two stimulus intensities: 2.2 x and 3.3 x detection threshold), and after non-painful acoustical stimulation of the right ear. The subjects rated the perceived intensity of the painful stimuli by means of a visual analogue scale. In addition the spontaneous EEG was analysed in the frequency domain and the vigilance of the subjects was assessed in a tracking task. 3. Diazepam reduced significantly the amplitudes of the event-related potentials after painful stimulation of the tooth pulp and after acoustical stimulation. In contrast only a small, statistically non-significant reduction could be found after painful stimulation with CO2. The pain ratings of the painful stimuli were not affected by diazepam. Diazepam reduced the performance of the tracking task. A decrease of arousal could be found in the alpha 2-range, whereas in the beta 2 and the theta-range the power density increased under diazepam. 4. We demonstrated that event-related potentials after painful stimulation of the nasal mucosa with CO2 are less affected by the nonspecific effects of the tranquilizer diazepam than event-related potentials after painful stimulation of the tooth pulp. The effects of diazepam on the tracking task, the spontaneous EEG and the event-related potentials clearly confirm its sedative properties. Diazepam had no analgesic effect measurable by pain intensity estimates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bromm B., Meier W., Scharein E. Pre-stimulus/post-stimulus relations in EEG spectra and their modulations by an opioid and an antidepressant. Electroencephalogr Clin Neurophysiol. 1989 Sep;73(3):188–197. doi: 10.1016/0013-4694(89)90119-3. [DOI] [PubMed] [Google Scholar]
- Chapman C. R., Schimek F., Gehrig J. D., Gerlach R., Colpitts Y. H. Effects of nitrous oxide, transcutaneous electrical stimulation, and their combination on brain potentials elicited by painful stimulation. Anesthesiology. 1983 Mar;58(3):250–256. doi: 10.1097/00000542-198303000-00009. [DOI] [PubMed] [Google Scholar]
- Chen A. C., Chapman C. R. Aspirin analgesia evaluated by event-related potentials in man: possible central action in brain. Exp Brain Res. 1980;39(4):359–364. doi: 10.1007/BF00239300. [DOI] [PubMed] [Google Scholar]
- Chudler E. H., Dong W. K., Kawakami Y. Tooth pulp-evoked potentials in the monkey: cortical surface and intracortical distribution. Pain. 1985 Jul;22(3):221–233. doi: 10.1016/0304-3959(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Eccles R., Jawad M. S., Morris S. Olfactory and trigeminal thresholds and nasal resistance to airflow. Acta Otolaryngol. 1989 Sep-Oct;108(3-4):268–273. doi: 10.3109/00016488909125527. [DOI] [PubMed] [Google Scholar]
- Handwerker H. O., Kobal G. Psychophysiology of experimentally induced pain. Physiol Rev. 1993 Jul;73(3):639–671. doi: 10.1152/physrev.1993.73.3.639. [DOI] [PubMed] [Google Scholar]
- Hari R., Kaukoranta E., Reinikainen K., Huopaniemie T., Mauno J. Neuromagnetic localization of cortical activity evoked by painful dental stimulation in man. Neurosci Lett. 1983 Nov 21;42(1):77–82. doi: 10.1016/0304-3940(83)90425-1. [DOI] [PubMed] [Google Scholar]
- Harkins S. W., Benedetti C., Colpitts Y. H., Chapman C. R. Effects of nitrous oxide inhalation on brain potentials evoked by auditory and noxious dental stimulation. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6(2):167–174. doi: 10.1016/s0278-5846(82)80192-9. [DOI] [PubMed] [Google Scholar]
- Hummel T., Friedmann T., Pauli E., Niebch G., Borbe H. O., Kobal G. Dose-related analgesic effects of flupirtine. Br J Clin Pharmacol. 1991 Jul;32(1):69–76. doi: 10.1111/j.1365-2125.1991.tb05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J., Kobal G., Kaukoranta E., Hari R. Cortical responses to painful CO2 stimulation of nasal mucosa; a magnetoencephalographic study in man. Electroencephalogr Clin Neurophysiol. 1986 Oct;64(4):347–349. doi: 10.1016/0013-4694(86)90159-8. [DOI] [PubMed] [Google Scholar]
- Kobal G., Hummel C., Nuernberg B., Brune K. Effects of pentazocine and acetylsalicylic acid on pain-rating, pain-related evoked potentials and vigilance in relationship to pharmacokinetic parameters. Agents Actions. 1990 Mar;29(3-4):342–359. doi: 10.1007/BF01966467. [DOI] [PubMed] [Google Scholar]
- Kobal G. Pain-related electrical potentials of the human nasal mucosa elicited by chemical stimulation. Pain. 1985 Jun;22(2):151–163. doi: 10.1016/0304-3959(85)90175-7. [DOI] [PubMed] [Google Scholar]
- Miltner W., Johnson R., Jr, Braun C., Larbig W. Somatosensory event-related potentials to painful and non-painful stimuli: effects of attention. Pain. 1989 Sep;38(3):303–312. doi: 10.1016/0304-3959(89)90217-0. [DOI] [PubMed] [Google Scholar]
- Miltner W., Larbig W., Braun C. Biofeedback of somatosensory event-related potentials: can individual pain sensations be modified by biofeedback-induced self-control of event-related potentials? Pain. 1988 Nov;35(2):205–213. doi: 10.1016/0304-3959(88)90228-X. [DOI] [PubMed] [Google Scholar]
- Raab W. H., Reithmayer K., Müller H. F. Ein Verfahren zur Testung von Lokalanästhetika. Dtsch Zahnarztl Z. 1990 Oct;45(10):629–632. [PubMed] [Google Scholar]
- WALTER W. G., COOPER R., ALDRIDGE V. J., MCCALLUM W. C., WINTER A. L. CONTINGENT NEGATIVE VARIATION: AN ELECTRIC SIGN OF SENSORIMOTOR ASSOCIATION AND EXPECTANCY IN THE HUMAN BRAIN. Nature. 1964 Jul 25;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wysocki C. J., Beauchamp G. K. Ability to smell androstenone is genetically determined. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4899–4902. doi: 10.1073/pnas.81.15.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre R. J., Jones-Gotman M. Right-nostril advantage for discrimination of odors. Percept Psychophys. 1990 Jun;47(6):526–531. doi: 10.3758/bf03203105. [DOI] [PubMed] [Google Scholar]


