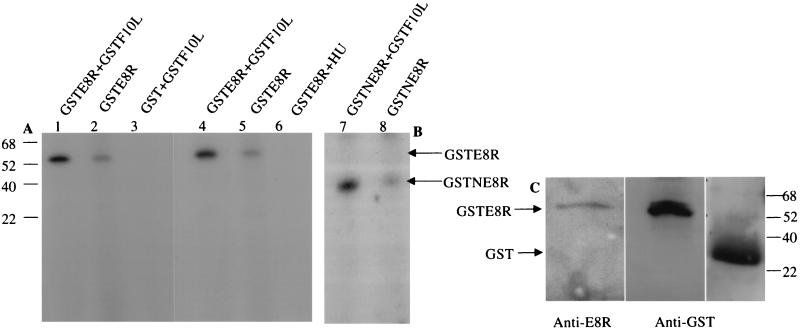
FIG. 7.
E8R is phosphorylated in vitro by the F10L kinase. GSTE8R was expressed in HeLa cells by infection with VV-T7 followed by transfection of pTMGSTE8R. GST or GSTF10L was expressed in and purified from E. coli. (A) Purified GSTE8R plus GSTF10L (lane 1), GSTE8R alone (lane 2), and GST plus GSTF10L (lane 3) were incubated for 20 min at 37°C in the presence of [γ-32P]ATP and then subjected to SDS-PAGE. GSTE8R is phosphorylated by the F10L kinase (lane 1), whereas there is no band corresponding to GST (lane 3). Note that in the sample with GSTE8R alone (lane 2), a faint 55-kDa band can also be detected. Purified GSTE8R plus GSTF10L (lane 4), GSTE8R alone (lane 5), and GSTE8R isolated from cells infected and transfected in the presence of HU (lane 6) were incubated as described above. In the sample treated with HU, the band corresponding to GSTE8R cannot be detected. (B) GSTNE8R plus GSTF10L (lane 7) and GSTNE8R (lane 8) were incubated as described for panel A. Whereas a phosphorylated band corresponding to GSTNE8R can readily be detected in lane 7, only a faint band can be seen in the absence of the kinase in lane 8. (C) Corresponding Western blots of GSTE8R or GST isolated from infected and transfected cells or bacteria, respectively, with anti-GST and anti-E8R antibodies to show the expected sizes of the proteins. The numbers to the left of panel A and to the right of panel C indicate the positions of the molecular mass markers (in kilodaltons).