Abstract
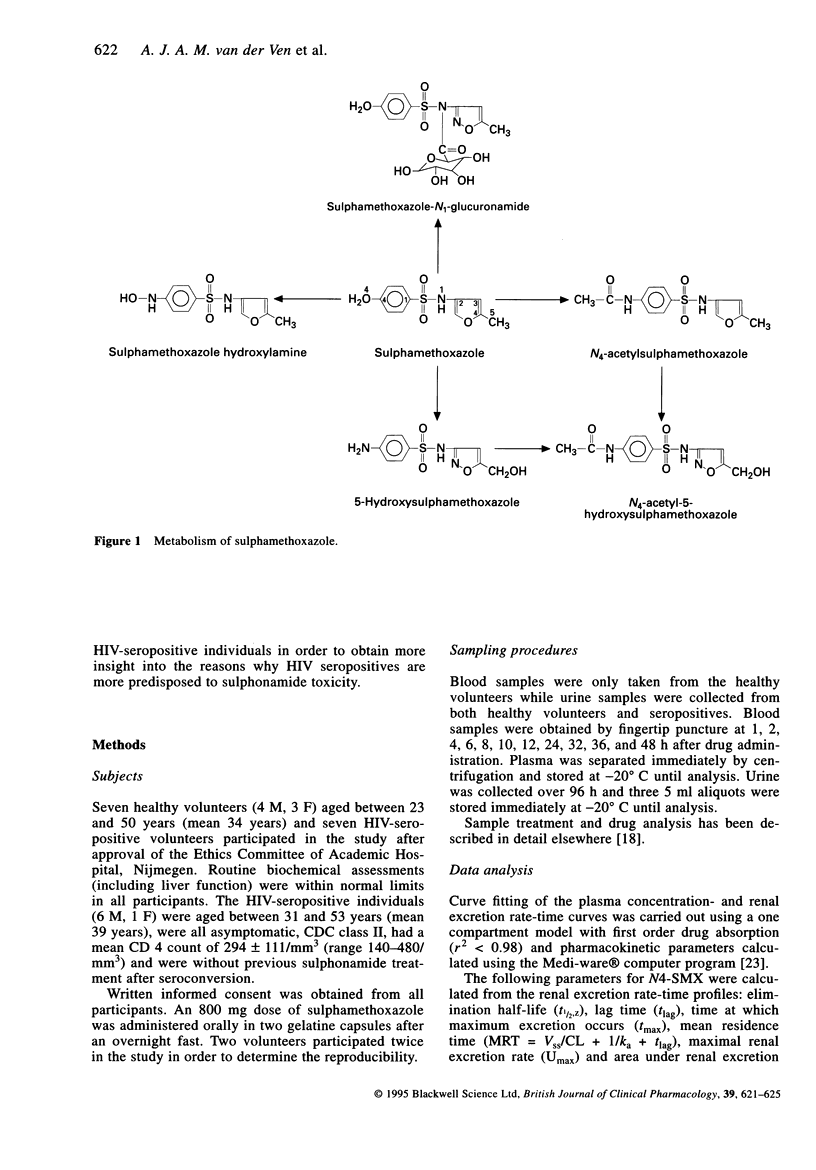
1. The urinary excretion of sulphamethoxazole and its metabolites was compared between healthy volunteers and HIV-seropositive patients in order to get a better understanding of why HIV seropositives are more predisposed to idiosyncratic toxicity of sulphonamides. 2. A single 800 mg oral dose of sulphamethoxazole was administered to seven healthy volunteers and seven asymptomatic HIV seropositives without previous use of sulphonamides. 3. Urine was collected for 4 days and drug analysis was by h.p.l.c. 4. No difference was observed between seropositive and seronegative individuals in the urinary recovery of sulphamethoxazole, N4-acetyl-, 5-hydroxy-, N4-acetyl-5-hydroxy-sulphamethoxazole and the N1-glucuronide conjugate. However the recovery of the hydroxylamine metabolite of sulphamethoxazole was significantly lower in the HIV seropositives (0.50 +/- 0.51 vs 2.23 +/- 0.85%; 95% CI on the difference, -0.90 to -2.55; P = 0.0006). 5. Sulphamethoxazole hydroxylamine may be a factor in the susceptibility of HIV infected individuals to sulphonamides.
Full text
PDF
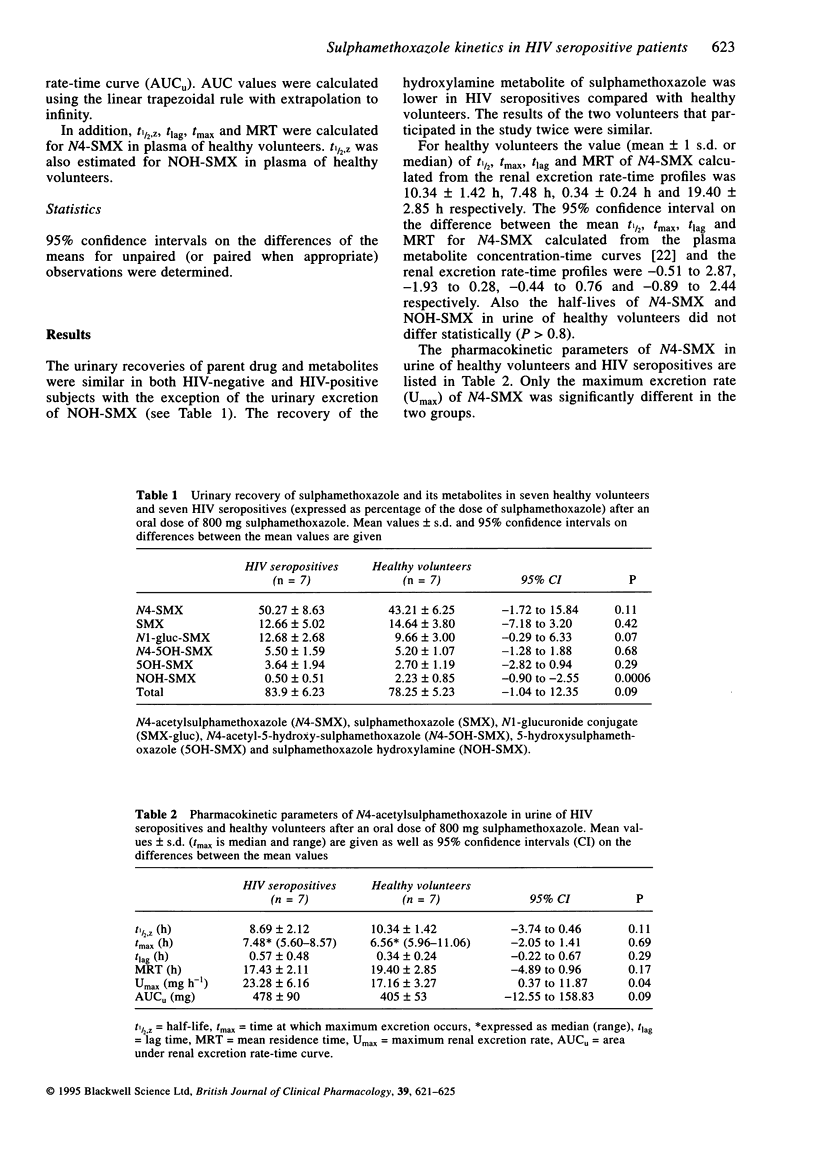


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden F. J., Harman P. J., Lucas C. R. Serum trimethoprim and sulphamethoxazole levels in AIDS. Lancet. 1986 Apr 12;1(8485):853–853. doi: 10.1016/s0140-6736(86)90958-x. [DOI] [PubMed] [Google Scholar]
- Buhl R., Jaffe H. A., Holroyd K. J., Wells F. B., Mastrangeli A., Saltini C., Cantin A. M., Crystal R. G. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989 Dec 2;2(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- Cribb A. E., Miller M., Leeder J. S., Hill J., Spielberg S. P. Reactions of the nitroso and hydroxylamine metabolites of sulfamethoxazole with reduced glutathione. Implications for idiosyncratic toxicity. Drug Metab Dispos. 1991 Sep-Oct;19(5):900–906. [PubMed] [Google Scholar]
- Cribb A. E., Spielberg S. P. Sulfamethoxazole is metabolized to the hydroxylamine in humans. Clin Pharmacol Ther. 1992 May;51(5):522–526. doi: 10.1038/clpt.1992.57. [DOI] [PubMed] [Google Scholar]
- Eck H. P., Gmünder H., Hartmann M., Petzoldt D., Daniel V., Dröge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol Chem Hoppe Seyler. 1989 Feb;370(2):101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- Gordin F. M., Simon G. L., Wofsy C. B., Mills J. Adverse reactions to trimethoprim-sulfamethoxazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Apr;100(4):495–499. doi: 10.7326/0003-4819-100-4-495. [DOI] [PubMed] [Google Scholar]
- Jaffe H. S., Abrams D. I., Ammann A. J., Lewis B. J., Golden J. A. Complications of co-trimoxazole in treatment of AIDS-associated Pneumocystis carinii pneumonia in homosexual men. Lancet. 1983 Nov 12;2(8359):1109–1111. doi: 10.1016/s0140-6736(83)90627-x. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Hiemenz J. W., Macher A. M., Stover D., Murray H. W., Shelhamer J., Lane H. C., Urmacher C., Honig C., Longo D. L. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984 May;100(5):663–671. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Medina I., Benowitz N. L., Jacob P., 3rd, Wofsy C. B., Mills J., 5th Dapsone, trimethoprim, and sulfamethoxazole plasma levels during treatment of Pneumocystis pneumonia in patients with the acquired immunodeficiency syndrome (AIDS). Evidence of drug interactions. Ann Intern Med. 1989 Apr 15;110(8):606–611. doi: 10.7326/0003-4819-110-8-606. [DOI] [PubMed] [Google Scholar]
- Medina I., Mills J., Leoung G., Hopewell P. C., Lee B., Modin G., Benowitz N., Wofsy C. B. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990 Sep 20;323(12):776–782. doi: 10.1056/NEJM199009203231202. [DOI] [PubMed] [Google Scholar]
- Proost J. H., Meijer D. K. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med. 1992 May;22(3):155–163. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- Rieder M. J., Uetrecht J., Shear N. H., Cannon M., Miller M., Spielberg S. P. Diagnosis of sulfonamide hypersensitivity reactions by in-vitro "rechallenge" with hydroxylamine metabolites. Ann Intern Med. 1989 Feb 15;110(4):286–289. doi: 10.7326/0003-4819-110-4-286. [DOI] [PubMed] [Google Scholar]
- Rieder M. J., Uetrecht J., Shear N. H., Spielberg S. P. Synthesis and in vitro toxicity of hydroxylamine metabolites of sulfonamides. J Pharmacol Exp Ther. 1988 Feb;244(2):724–728. [PubMed] [Google Scholar]
- Sattler F. R., Cowan R., Nielsen D. M., Ruskin J. Trimethoprim-sulfamethoxazole compared with pentamidine for treatment of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective, noncrossover study. Ann Intern Med. 1988 Aug 15;109(4):280–287. doi: 10.7326/0003-4819-109-4-280. [DOI] [PubMed] [Google Scholar]
- Shear N. H., Spielberg S. P. In vitro evaluation of a toxic metabolite of sulfadiazine. Can J Physiol Pharmacol. 1985 Nov;63(11):1370–1372. doi: 10.1139/y85-225. [DOI] [PubMed] [Google Scholar]
- Small C. B., Harris C. A., Friedland G. H., Klein R. S. The treatment of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Arch Intern Med. 1985 May;145(5):837–840. [PubMed] [Google Scholar]
- Vree T. B., Hekster Y. A. Clinical pharmacokinetics of sulfonamides and their metabolites: an encyclopedia. Antibiot Chemother (1971) 1987;37:1–214. [PubMed] [Google Scholar]
- Vree T. B., van der Ven A. J., Verwey-van Wissen C. P., van Ewijk-Beneken Kolmer E. W., Swolfs A. E., van Galen P. M., Amatdjais-Groenen H. Isolation, identification and determination of sulfamethoxazole and its known metabolites in human plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994 Aug 19;658(2):327–340. doi: 10.1016/0378-4347(94)00232-0. [DOI] [PubMed] [Google Scholar]
- Wharton J. M., Coleman D. L., Wofsy C. B., Luce J. M., Blumenfeld W., Hadley W. K., Ingram-Drake L., Volberding P. A., Hopewell P. C. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective randomized trial. Ann Intern Med. 1986 Jul;105(1):37–44. doi: 10.7326/0003-4819-105-1-37. [DOI] [PubMed] [Google Scholar]
- van der Ven A. J., Koopmans P. P., Vree T. B., van der Meer J. W. Adverse reactions to co-trimoxazole in HIV infection. Lancet. 1991 Aug 17;338(8764):431–433. doi: 10.1016/0140-6736(91)91046-w. [DOI] [PubMed] [Google Scholar]
- van der Ven A. J., Mantel M. A., Vree T. B., Koopmans P. P., van der Meer J. W. Formation and elimination of sulphamethoxazole hydroxylamine after oral administration of sulphamethoxazole. Br J Clin Pharmacol. 1994 Aug;38(2):147–150. doi: 10.1111/j.1365-2125.1994.tb04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



