Abstract
1. Less than 5% of a dose of the conventional oral formulation of cyclosporin, Sandimmun, is absorbed in liver transplant recipients with external biliary drainage, necessitating intravenous administration of the drug and exposing the patient to increased risk of severe side-effects. 2. We compared the pharmacokinetics of the conventional oral formulation of cyclosporin with that of the new microemulsion formulation, Neoral, in eight liver transplant recipients with external biliary diversion. Patients were maintained on a continuous infusion of cyclosporin until steady-state conditions had been achieved. They were then given a test dose (10 mg kg-1) of either the conventional or microemulsion formulation (randomised order) followed by the same dose of the other formulation. Parent cyclosporin concentrations were measured in whole blood samples collected at timed intervals over the 24 h after the oral doses and pharmacokinetic parameters calculated. 3. The bioavailability of cyclosporin from the microemulsion formulation was, on average, 6.5-fold (95% C.I. 1.9 to 11.1-fold) greater than that of the conventional formulation, indicating the improved absorption characteristics of the new oral microemulsion formulation during external bile drainage. 4. A significant negative correlation was found between the external bile drainage volume and bioavailability of cyclosporin from the microemulsion formulation (r = -0.8; P = 0.016), suggesting that variability in cyclosporin absorption from the microemulsion formulation may still be at least partly attributable to bile- dependence.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
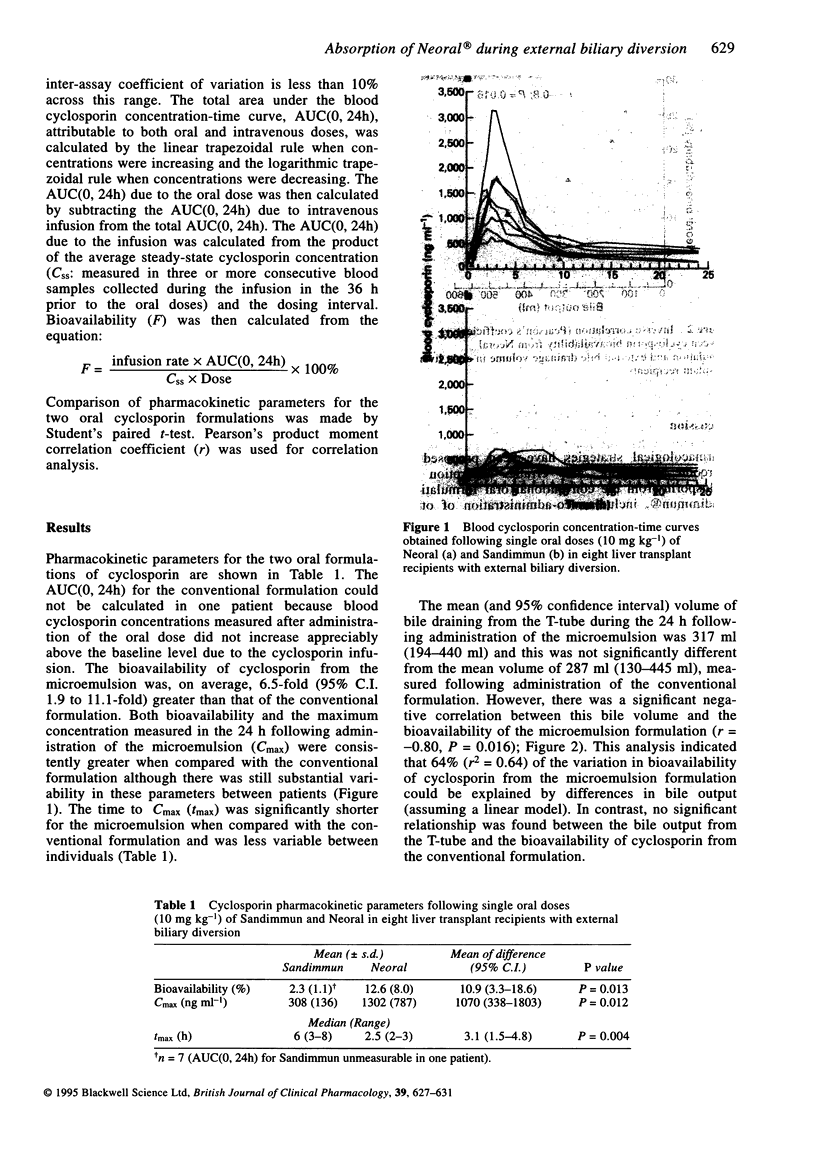
PDF

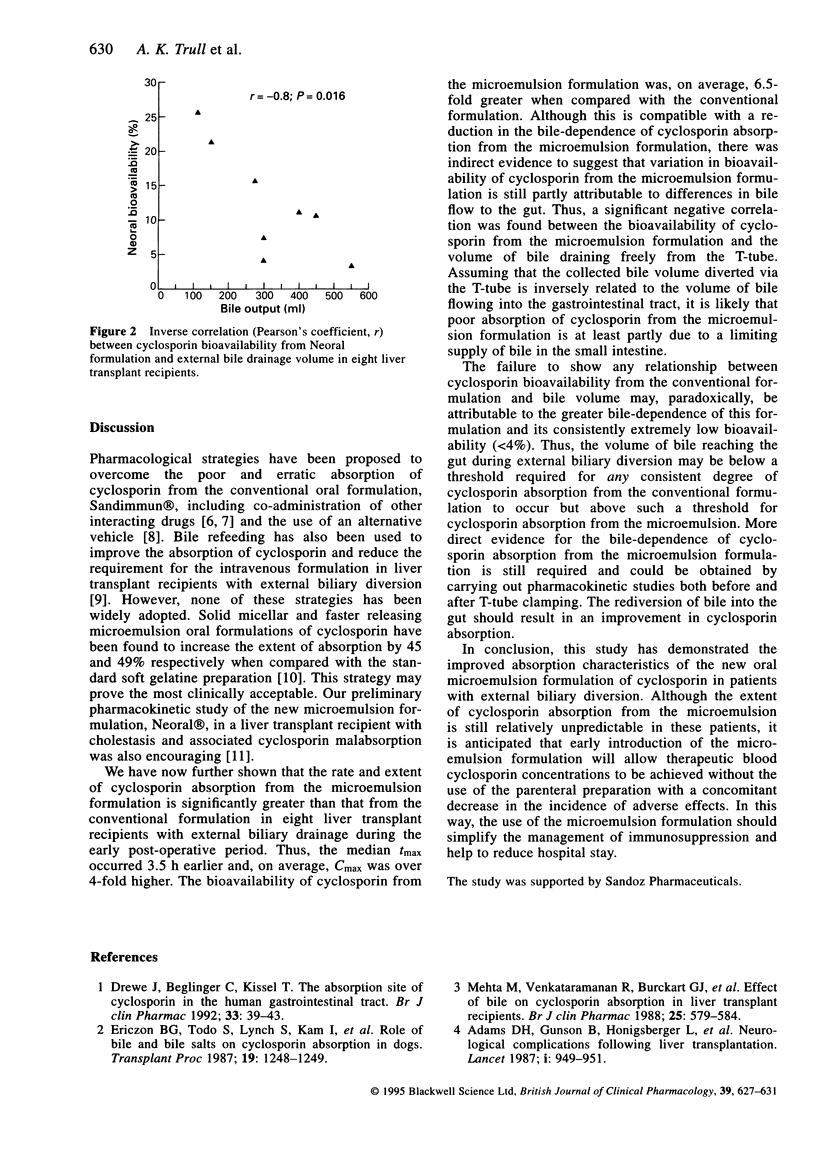

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Ponsford S., Gunson B., Boon A., Honigsberger L., Williams A., Buckels J., Elias E., McMaster P. Neurological complications following liver transplantation. Lancet. 1987 Apr 25;1(8539):949–951. doi: 10.1016/s0140-6736(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Brat D. J., Windebank A. J., Brimijoin S. Emulsifier for intravenous cyclosporin inhibits neurite outgrowth, causes deficits in rapid axonal transport and leads to structural abnormalities in differentiating N1E.115 neuroblastoma. J Pharmacol Exp Ther. 1992 May;261(2):803–810. [PubMed] [Google Scholar]
- Butman S. M., Wild J. C., Nolan P. E., Fagan T. C., Finley P. R., Hicks M. J., Mackie M. J., Copeland J. G., 3rd Prospective study of the safety and financial benefit of ketoconazole as adjunctive therapy to cyclosporine after heart transplantation. J Heart Lung Transplant. 1991 May-Jun;10(3):351–358. [PubMed] [Google Scholar]
- Drewe J., Beglinger C., Kissel T. The absorption site of cyclosporin in the human gastrointestinal tract. Br J Clin Pharmacol. 1992 Jan;33(1):39–43. doi: 10.1111/j.1365-2125.1992.tb03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe J., Meier R., Vonderscher J., Kiss D., Posanski U., Kissel T., Gyr K. Enhancement of the oral absorption of cyclosporin in man. Br J Clin Pharmacol. 1992 Jul;34(1):60–64. doi: 10.1111/j.1365-2125.1992.tb04108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme M. P., Provenzano R., Dehoorne-Smith M., Edwards D. J. Trough concentrations of cyclosporine in blood following administration with grapefruit juice. Br J Clin Pharmacol. 1993 Nov;36(5):457–459. doi: 10.1111/j.1365-2125.1993.tb00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericzon B. G., Todo S., Lynch S., Kam I., Ptachcinski R. J., Burckart G. J., Van Thiel D. H., Starzl T. E., Venkataramanan R. Role of bile and bile salts on cyclosporine absorption in dogs. Transplant Proc. 1987 Feb;19(1 Pt 2):1248–1249. [PMC free article] [PubMed] [Google Scholar]
- Mehta M. U., Venkataramanan R., Burckart G. J., Ptachcinski R. J., Delamos B., Stachak S., Van Thiel D. H., Iwatsuki S., Starzl T. E. Effect of bile on cyclosporin absorption in liver transplant patients. Br J Clin Pharmacol. 1988 May;25(5):579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merion R. M., Gorski D. H., Burtch G. D., Turcotte J. G., Colletti L. M., Campbell D. A., Jr Bile refeeding after liver transplantation and avoidance of intravenous cyclosporine. Surgery. 1989 Oct;106(4):604–610. [PubMed] [Google Scholar]
- Sokol R. J., Johnson K. E., Karrer F. M., Narkewicz M. R., Smith D., Kam I. Improvement of cyclosporin absorption in children after liver transplantation by means of water-soluble vitamin E. Lancet. 1991 Jul 27;338(8761):212–214. doi: 10.1016/0140-6736(91)90349-t. [DOI] [PubMed] [Google Scholar]
- Trull A. K., Tan K. K., Uttridge J., Bauer T., Alexander G. J., Jamieson N. V. Cyclosporin absorption from microemulsion formulation in liver transplant recipient. Lancet. 1993 Feb 13;341(8842):433–433. doi: 10.1016/0140-6736(93)93025-v. [DOI] [PubMed] [Google Scholar]


