Abstract
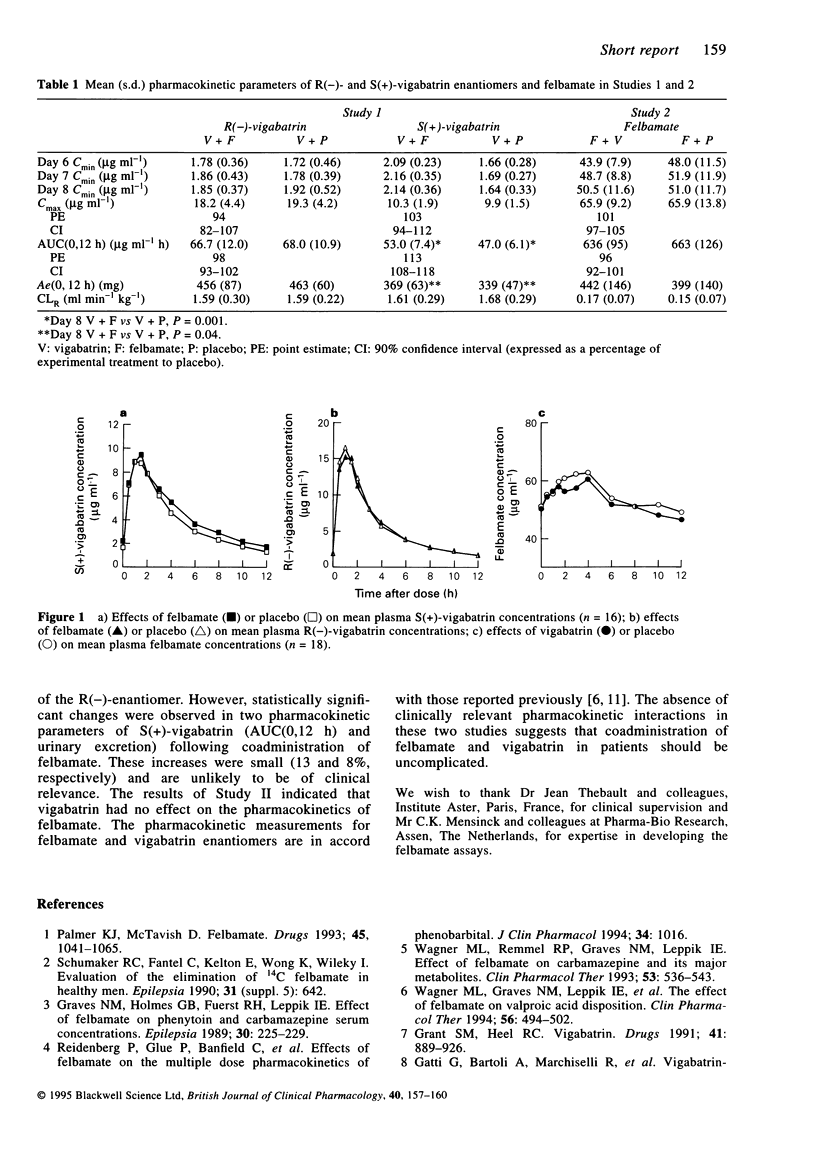
To assess the possible occurrence of pharmacokinetic interactions between the antiepileptic agents felbamate and vigabatrin, two randomized, double-blind, placebo-controlled, crossover studies were conducted in healthy male volunteers. In Study I, 18 subjects received oral vigabatrin 1000 mg every 12 h for two 8 day periods with felbamate 1200 mg every 12 h or placebo. In Study II, 18 other volunteers were administered oral felbamate 1200 mg every 12 h for two 8 day periods with vigabatrin 1000 mg every 12 h or placebo. On the eighth day of each treatment period, blood and urine samples were collected over 12 h for determination of the active S(+)- and inactive R(-)-vigabatrin enantiomer concentrations (Study I) or felbamate concentrations (Study II). In Study I, the pharmacokinetic parameters of R(-)-vigabatrin were similar during co-administration with felbamate or placebo. Felbamate produced a 13% increase in AUC(0,12 h) and an 8% increase in urinary excretion of S(+)-vigabatrin. Although these changes were statistically significant, their magnitude was small. In Study II, the pharmacokinetic parameters of felbamate were similar during concurrent administration with vigabatrin or placebo. These data indicate that there are no clinically relevant pharmacokinetic interactions between felbamate and vigabatrin in man
Keywords: felbamate, vigabatrin, pharmacokinetics
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grant S. M., Heel R. C. Vigabatrin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in epilepsy and disorders of motor control. Drugs. 1991 Jun;41(6):889–926. doi: 10.2165/00003495-199141060-00007. [DOI] [PubMed] [Google Scholar]
- Graves N. M., Holmes G. B., Fuerst R. H., Leppik I. E. Effect of felbamate on phenytoin and carbamazepine serum concentrations. Epilepsia. 1989 Mar-Apr;30(2):225–229. doi: 10.1111/j.1528-1157.1989.tb05458.x. [DOI] [PubMed] [Google Scholar]
- Palmer K. J., McTavish D. Felbamate. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in epilepsy. Drugs. 1993 Jun;45(6):1041–1065. doi: 10.2165/00003495-199345060-00008. [DOI] [PubMed] [Google Scholar]
- Rey E., Pons G., Olive G. Vigabatrin. Clinical pharmacokinetics. Clin Pharmacokinet. 1992 Oct;23(4):267–278. doi: 10.2165/00003088-199223040-00003. [DOI] [PubMed] [Google Scholar]
- Rey E., Pons G., Richard M. O., Vauzelle F., D'Athis P., Chiron C., Dulac O., Beaumont D., Olive G. Pharmacokinetics of the individual enantiomers of vigabatrin (gamma-vinyl GABA) in epileptic children. Br J Clin Pharmacol. 1990 Aug;30(2):253–257. doi: 10.1111/j.1365-2125.1990.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. L., Graves N. M., Leppik I. E., Remmel R. P., Shumaker R. C., Ward D. L., Perhach J. L. The effect of felbamate on valproic acid disposition. Clin Pharmacol Ther. 1994 Nov;56(5):494–502. doi: 10.1038/clpt.1994.170. [DOI] [PubMed] [Google Scholar]
- Wagner M. L., Remmel R. P., Graves N. M., Leppik I. E. Effect of felbamate on carbamazepine and its major metabolites. Clin Pharmacol Ther. 1993 May;53(5):536–543. doi: 10.1038/clpt.1993.67. [DOI] [PubMed] [Google Scholar]


