Abstract
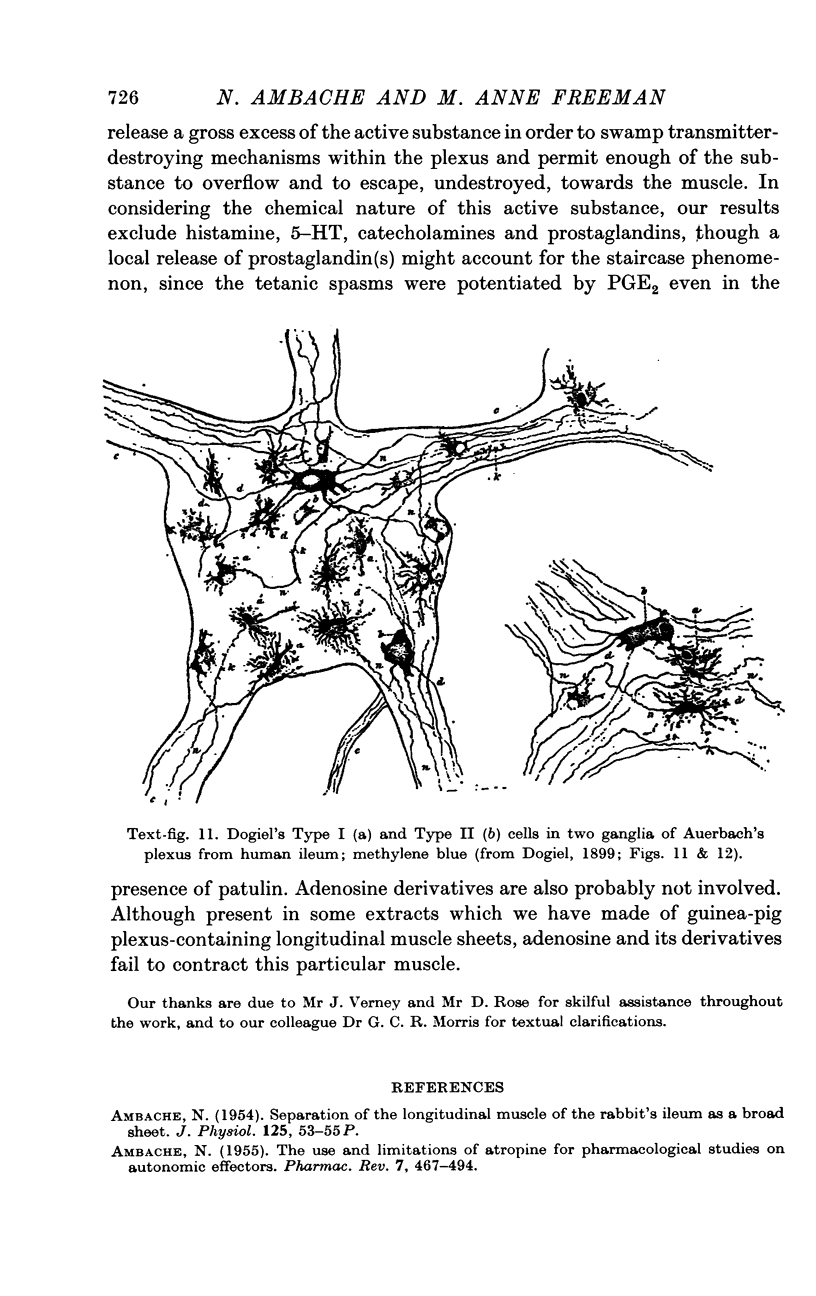
1. In accordance with the dual histology of Auerbach's plexus (Dogiel, 1899; Hill, 1927) two types of neurone can be shown to be humorally active in plexus-containing preparations of longitudinal muscle from guinea-pig ileum, taken at measured distances up to 95 cm above the ileocaecal valve, when such preparations are stimulated electrically under different conditions.
2. The rapid twitch, lasting 3-8 sec, which is elicited by single shocks of 0·1 or 0·2 msec pulse width, and the effect of 5-15 ng doses of acetylcholine which matched this twitch, were both extinguished equally effectively and completely by atropine sulphate (0·4-1 × 10-8 g/ml.) or by hyoscine hydrobromide. This twitch-response is therefore caused by an excitation of cholinergic motor neurones of normal susceptibility to atropine. These are believed to be the Dogiel (1899) Type II cells of Auerbach's plexus, as suggested by Hill (1927).
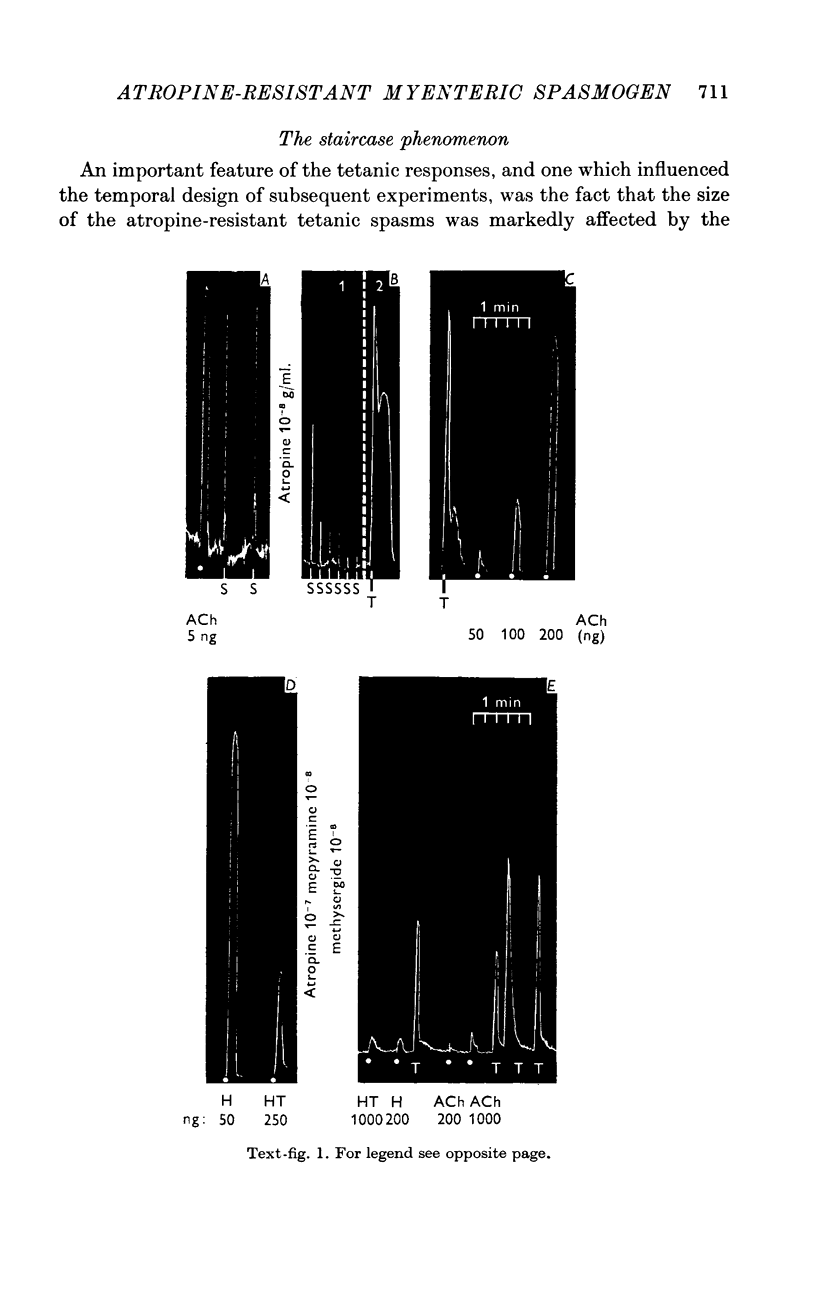
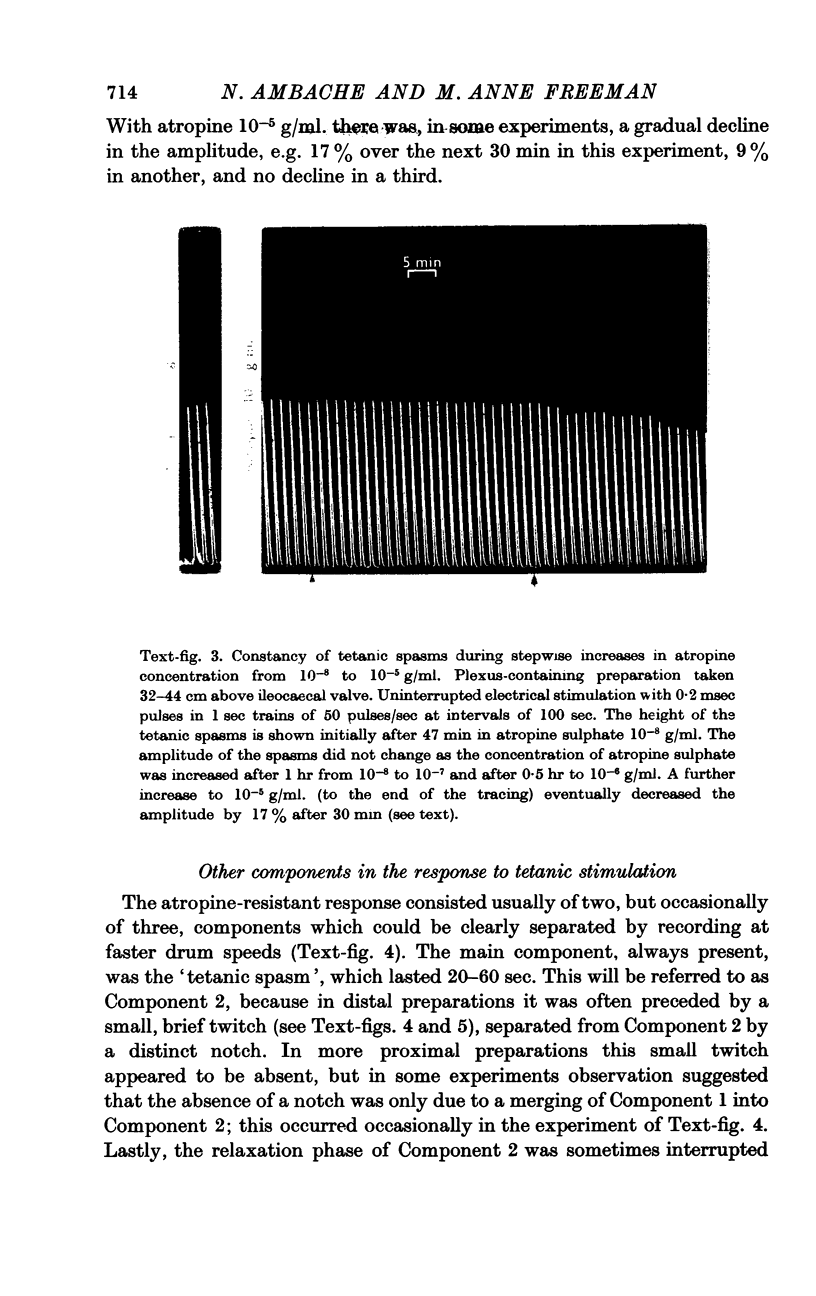
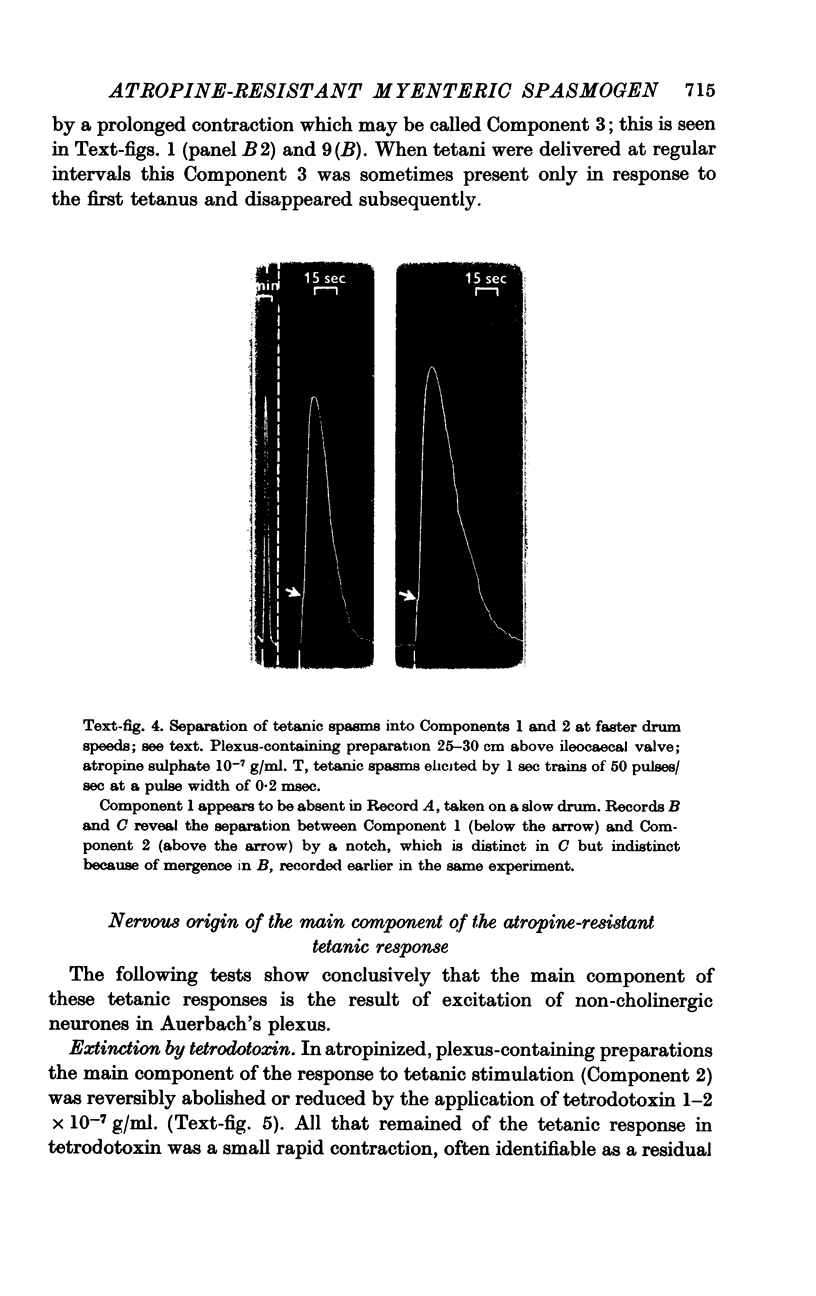
3. After extinction of the twitch by the invariably effective atropineblock, a second type of muscle response was revealed by tetanic stimulation with 1 sec trains of 50 pulses of the same voltage and of pulse width preferably 0·2 msec. The tetanic responses consisted of spasms of longer delay and duration (20-60 sec). These spasms could be matched by doses of acetylcholine of the order of 200 ng. However, if the atropine concentration was now raised to 10-7, or even 10-6 g/ml., the effect of 200-1000 ng of acetylcholine was abolished, but the tetanic spasms persisted without decrease in amplitude. In other experiments the height of the spasms remained constant as the concentration of atropine sulphate was raised from 10-8 to 10-6 g/ml. and was only slightly decreased by 10-5 g/ml. Hence, these tetanic contractions are not due to a surmounting of the atropine-block by the increased release of acetylcholine following the 50 pulses.
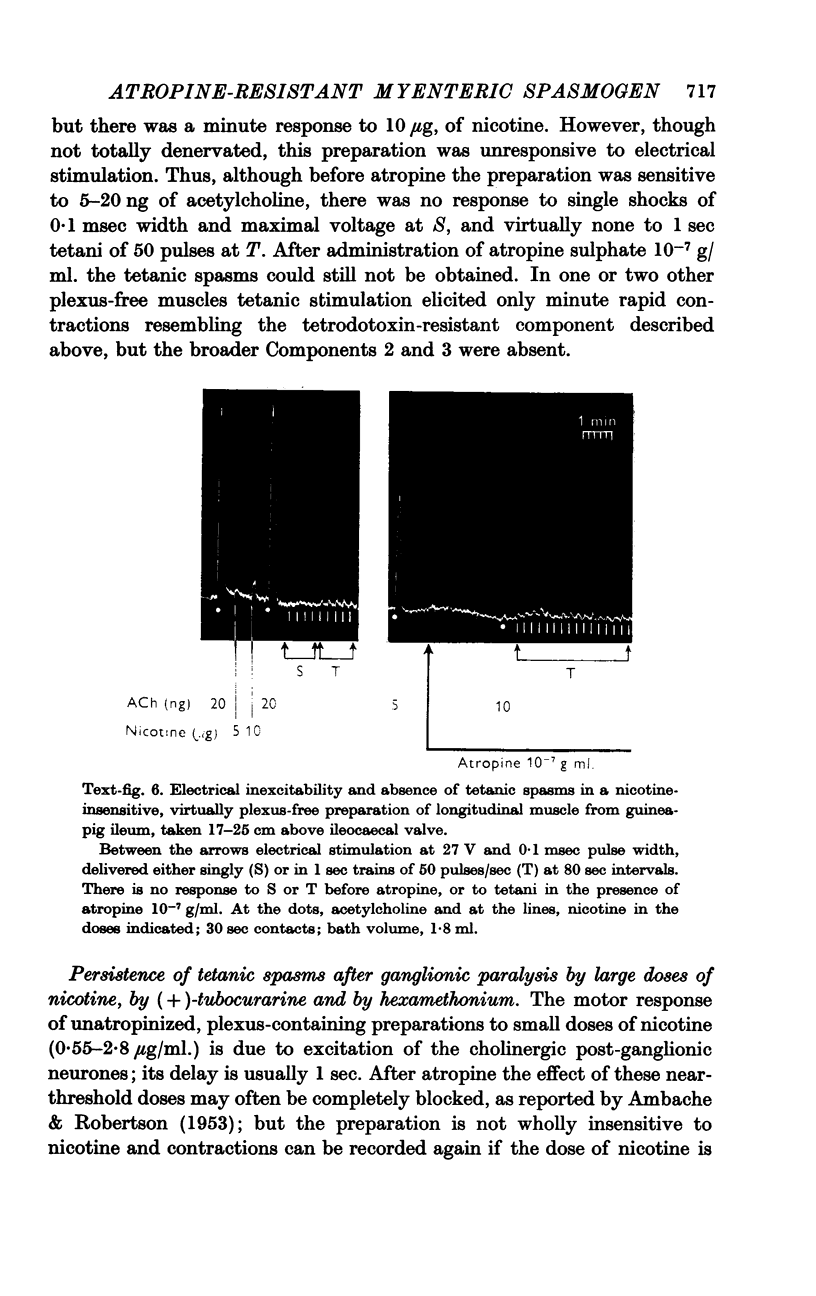
4. The tetanic spasms originate from excitation of non-cholinergic neurones, perhaps the associative Dogiel Type I cells of Auerbach's plexus (Hill, 1927), since the spasms were abolished reversibly by tetrodotoxin 2 × 10-7 g/ml. and were absent from plexus-free, nicotine-insensitive preparations of the longitudinal muscle, both before and after atropinization.
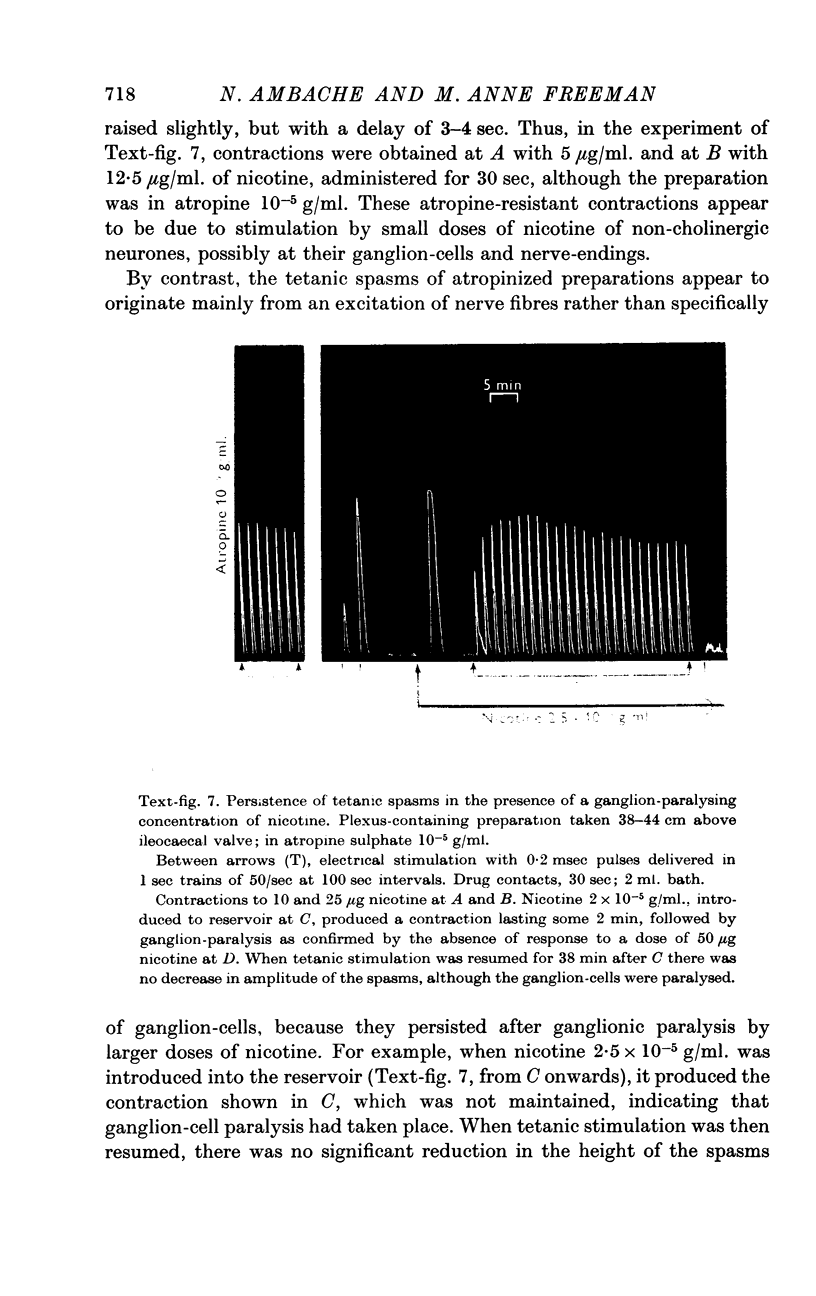
5. The tetanic spasms were not reduced by ganglion-block with paralysing doses of nicotine, with (+)-tubocurarine or with hexamethonium.
6. The tetanic spasms are not mediated by a release of 5-hydroxytryptamine (5-HT) or of histamine, since they persisted in concentrations of methysergide and mepyramine adequate to block matching doses of histamine or 5-HT, or multiples thereof. Catecholamines were also excluded.
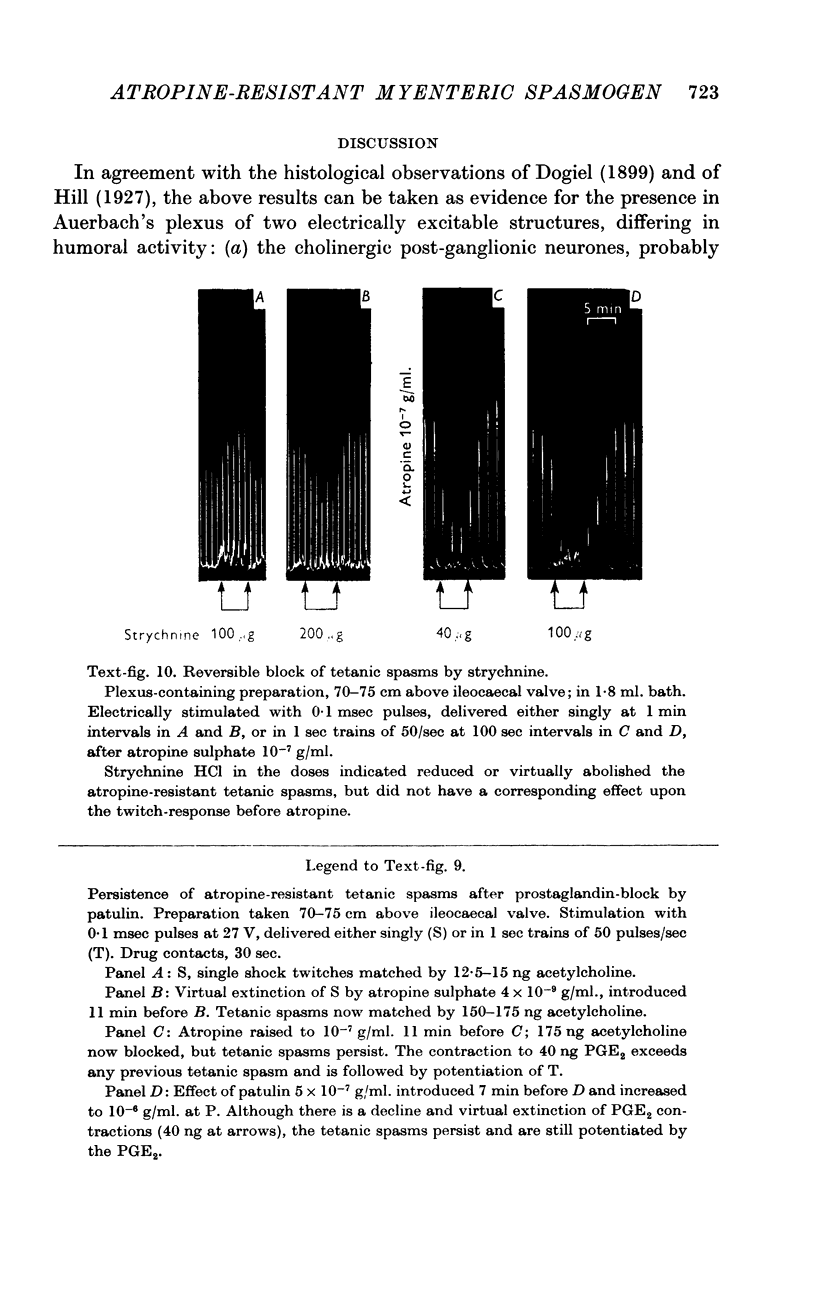
7. The tetanic spasms are not mediated by a release of a prostaglandin, because they were not reduced by 0·5-2 × 10-6 g/ml. of patulin (Ambache, 1957), which blocked the contractions evoked by matching doses of prostaglandins PGE2 or PGF2α; even after this block, PGE2 still potentiated subsequent tetanic responses.
8. The tetanic spasms were reduced or virtually abolished by strychnine in concentrations which did not depress the twitch.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N., EDWARDS J. Reversal of nicotine action on the intestine by atropine. Br J Pharmacol Chemother. 1951 Jun;6(2):311–317. doi: 10.1111/j.1476-5381.1951.tb00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N. Further studies on the preparation, purification and nature of irin. J Physiol. 1959 May 19;146(2):255–294. doi: 10.1113/jphysiol.1959.sp006193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N., KAVANAGH L., WHITING J. EFFECT OF MECHANICAL STIMULATION ON RABBITS' EYES: RELEASE OF ACTIVE SUBSTANCE IN ANTERIOR CHAMBER PERFUSATES. J Physiol. 1965 Feb;176:378–408. doi: 10.1113/jphysiol.1965.sp007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N., ROBERTSON P. A. The nicotine-like actions of the 3-bromo-and 3:5-dibromo-phenyl ethers of choline (MBF and DBF). Br J Pharmacol Chemother. 1953 Jun;8(2):147–155. doi: 10.1111/j.1476-5381.1953.tb00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N. The unsaturated nature of irin and its interaction with lactones. J Physiol. 1958 Jan 23;140(1):24–5P. [PubMed] [Google Scholar]
- AMBACHE N. The use and limitations of atropine for pharmacological studies on autonomic effectors. Pharmacol Rev. 1955 Dec;7(4):467–494. [PubMed] [Google Scholar]
- Ambache N., Barsoum G. S. The release of histamine by isolated smooth muscles. J Physiol. 1939 Jul 14;96(2):139–145. doi: 10.1113/jphysiol.1939.sp003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMBRIDGE G. W., HOLGATE J. A. Superfusion as a method for the study of drug antagonism. Br J Pharmacol Chemother. 1955 Sep;10(3):326–335. doi: 10.1111/j.1476-5381.1955.tb00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H. H., Gaddum J. H. Reactions of denervated voluntary muscle, and their bearing on the mode of action of parasympathetic and related nerves. J Physiol. 1930 Sep 18;70(2):109–144. doi: 10.1113/jphysiol.1930.sp002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELIASSON R. The spasmolytic effect of patulin. Experientia. 1958 Dec 15;14(12):460–461. doi: 10.1007/BF02327377. [DOI] [PubMed] [Google Scholar]
- GADDUM J. H., PICARELLI Z. P. Two kinds of tryptamine receptor. Br J Pharmacol Chemother. 1957 Sep;12(3):323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNRO A. F. Effect of autonomic drugs on the responses of isolated preparations from the guinea-pig intestine to electrical stimulation. J Physiol. 1953 Apr 28;120(1-2):41–52. doi: 10.1113/jphysiol.1953.sp004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNRO A. F. The effect of adrenaline on the guinea-pig intestine. J Physiol. 1951 Jan;112(1-2):84–94. doi: 10.1113/jphysiol.1951.sp004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Schild H. O. The antagonism of disulphide polypeptides by thiols. Br J Pharmacol Chemother. 1965 Oct;25(2):418–431. doi: 10.1111/j.1476-5381.1965.tb02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON P. A. An antagonism of 5-hydroxytryptamine by atropine. J Physiol. 1953 Aug;121(2):54P–55P. [PubMed] [Google Scholar]