Abstract
1. The short-circuit current and absolute fluxes of Na+ and Cl- across the gastric mucosa of the 28-day rabbit foetus have been measured in vitro.
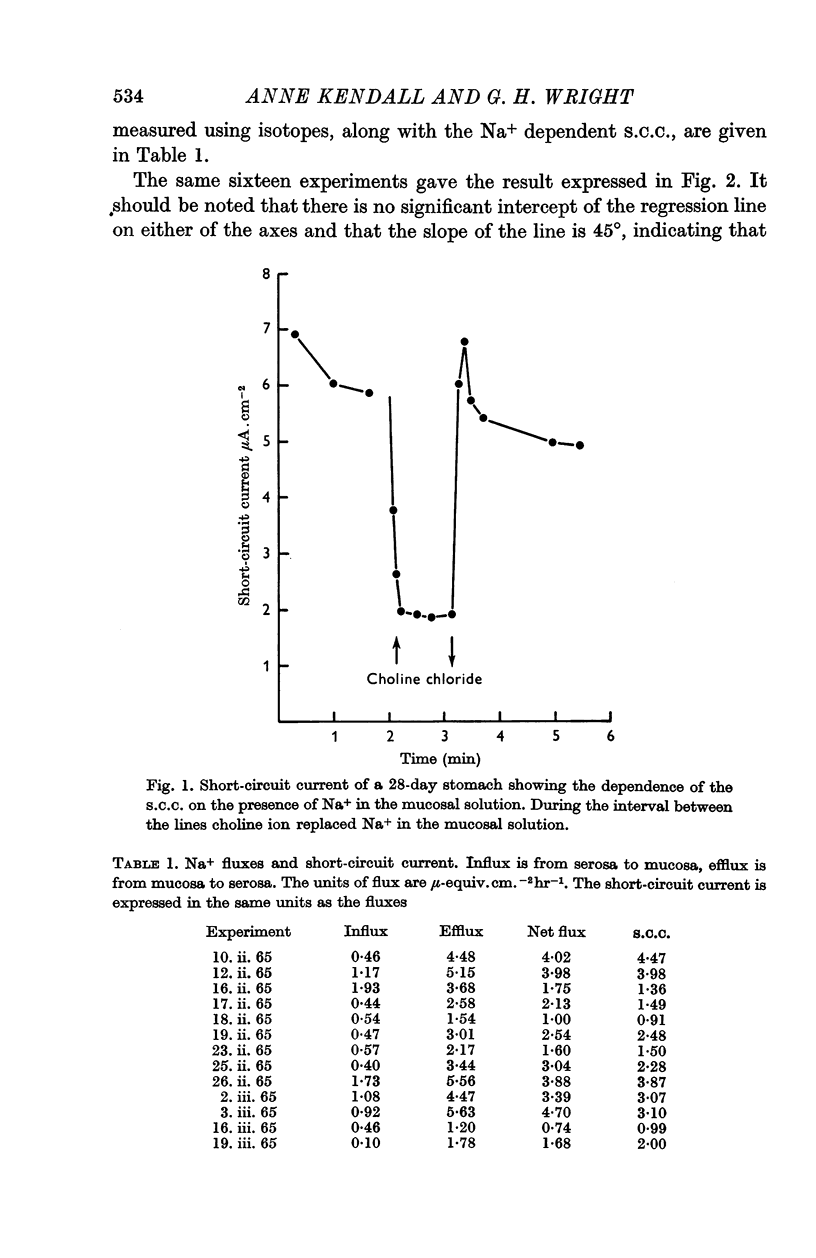
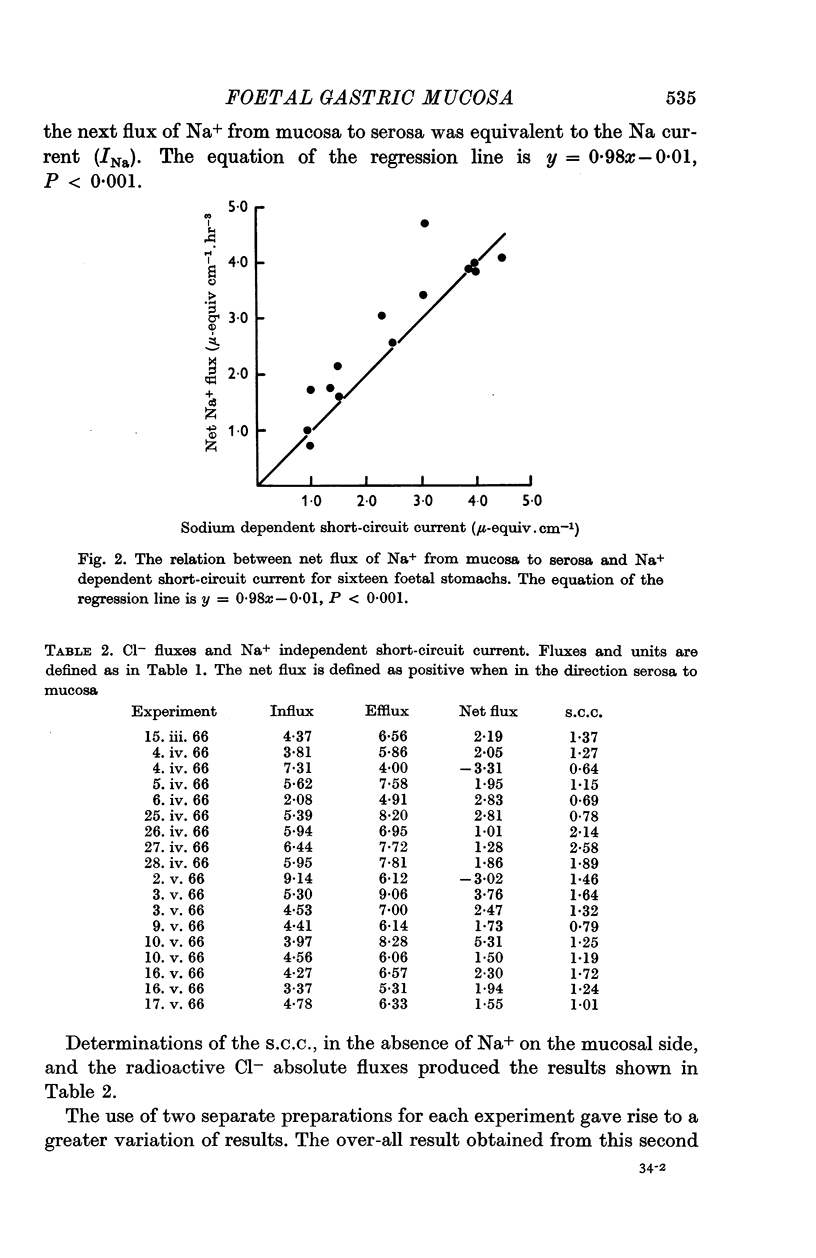
2. Substitution of Na+ in the solution bathing the mucosal surface by choline ion or K+ resulted in a 70% decrease in short-circuit current which was reversed when Na+ was restored to the mucosal solution. The portion of the short-circuit current dependent on the presence of Na+ in the mucosal solution was found to be equivalent to the net flux of Na+ from mucosa to serosa.
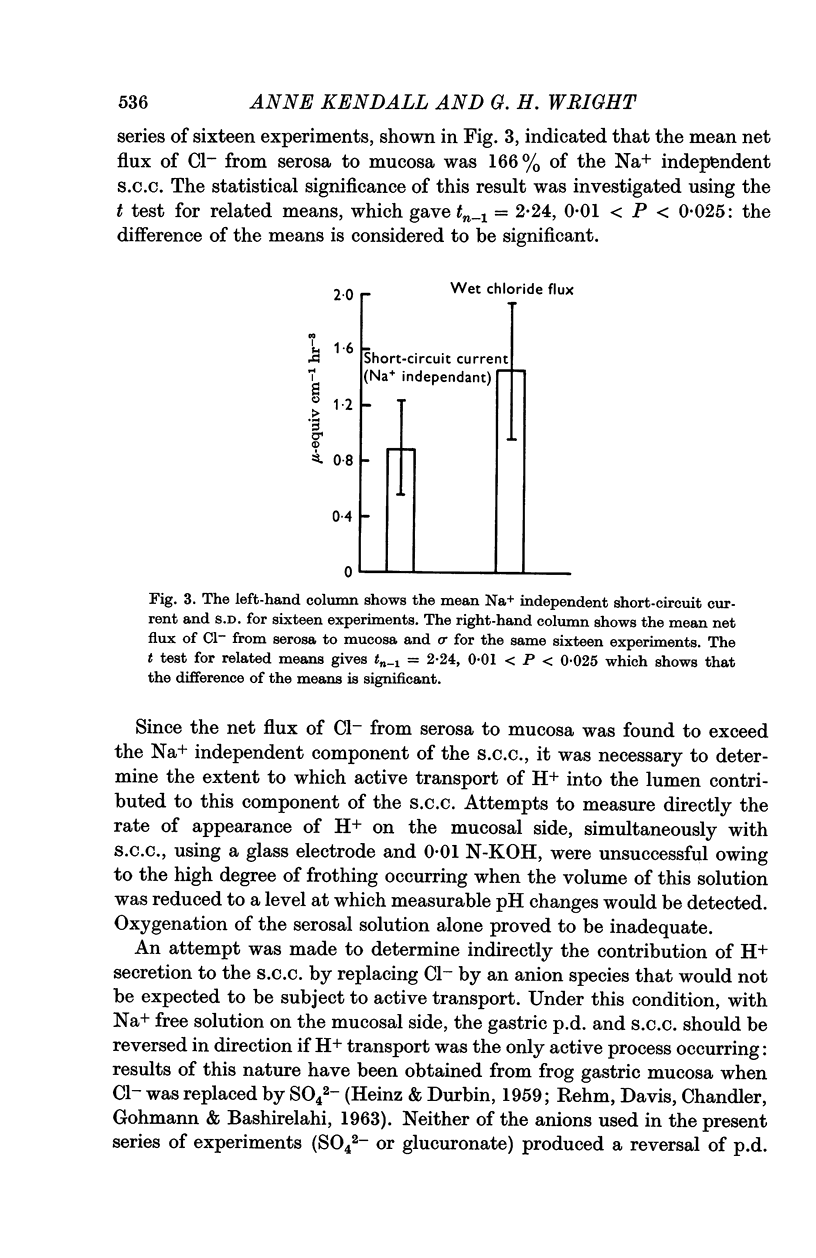
3. The net flux of Cl- from serosa to mucosa was compared with the short-circuit current persisting when Na+ had been replaced in the mucosal solution. Averaged results from sixteen experiments indicated that the net flux of Cl- was equivalent to 166% of the Na+ independent short-circuit current.
4. The results indicated that the component of short-circuit current associated with acid secretion was independent of the presence of Na+ in the mucosal solution.
5. The small scale of the experiments and the secretion of mucus by the preparation did not permit successful simultaneous measurement of H+ secretion and short-circuit current.
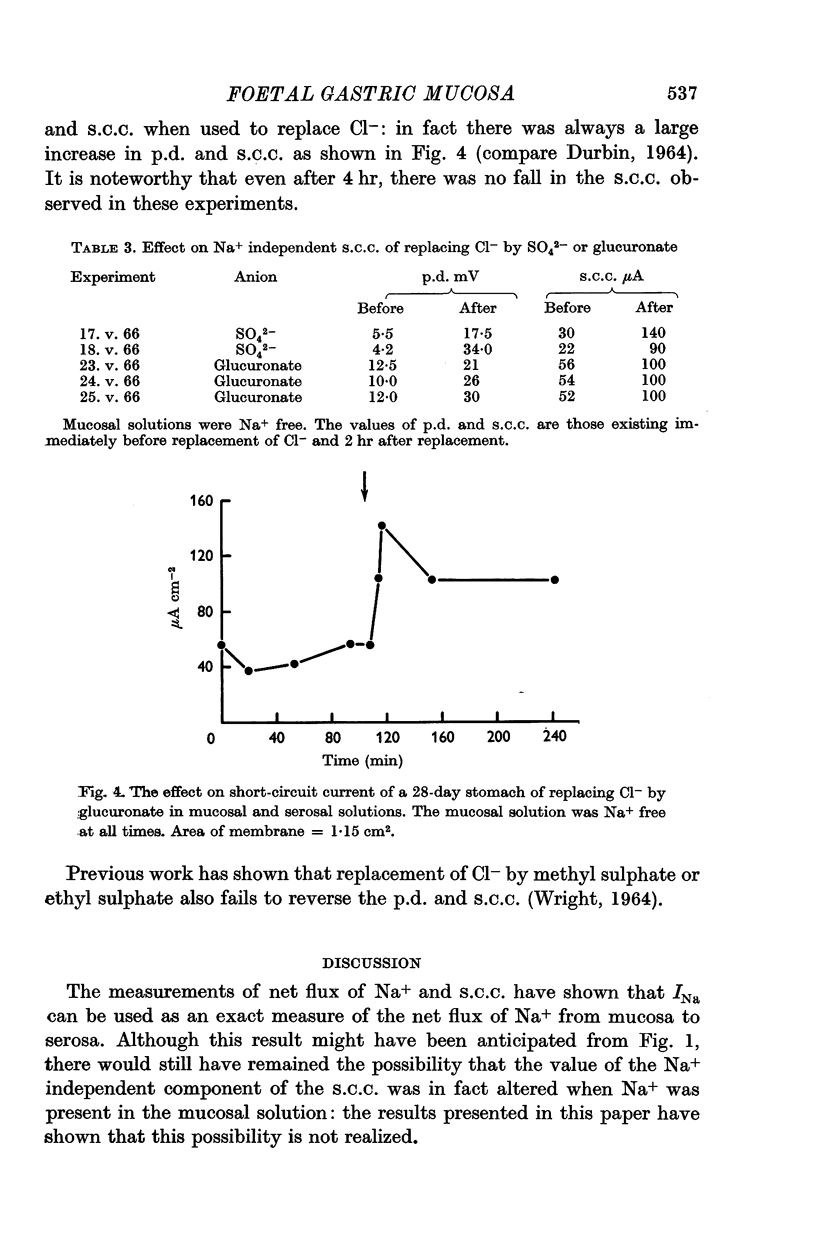
6. Replacement of Cl- by SO42- or glucuronate in the solutions on both sides did not result in a reversal or decrease in magnitude of the Na+ independent short-circuit current, even after allowing time for the tissue to become depleted of Cl-. It is suggested that a non-specific active anion transport was occurring.
Full text
PDF



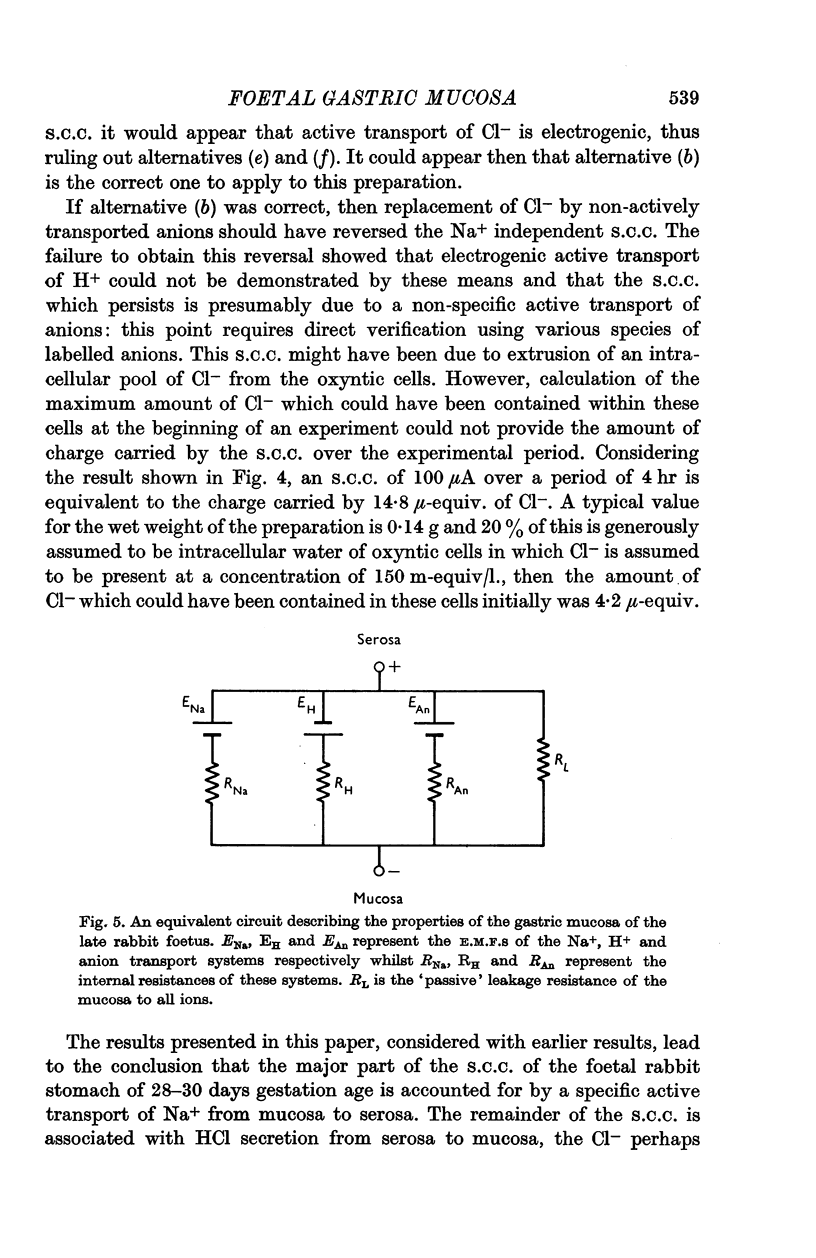

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN A. M., DENNIS W. H., REHM W. S. Movement of water, sodium, chloride and hydrogen ions across the resting stomach. Am J Physiol. 1959 Aug;197:332–336. doi: 10.1152/ajplegacy.1959.197.2.332. [DOI] [PubMed] [Google Scholar]
- DURBIN R. P. ANION REQUIREMENTS FOR GASTRIC ACID SECRETION. J Gen Physiol. 1964 Mar;47:735–748. doi: 10.1085/jgp.47.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRANT J. PERMEABILITY OF GUINEA-PIG SMOOTH MUSCLE TO NON-ELECTROLYTES. J Physiol. 1965 May;178:1–13. doi: 10.1113/jphysiol.1965.sp007610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINZ E., DURBIN R. Evidence for an independent hydrogen-ion pump in the stomach. Biochim Biophys Acta. 1959 Jan;31(1):246–247. doi: 10.1016/0006-3002(59)90461-5. [DOI] [PubMed] [Google Scholar]
- HOGBEN C. A. Active transport of chloride by isolated frog gastric epithelium; origin of the gastric mucosal potential. Am J Physiol. 1955 Mar;180(3):641–649. [PubMed] [Google Scholar]
- Kedem O., Essig A. Isotope flows and flux ratios in biological membranes. J Gen Physiol. 1965 Jul;48(6):1047–1070. doi: 10.1085/jgp.48.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REHM W. S., DAVIS T. L., CHANDLER C., GOHMANN E., Jr, BASHIRELAHI A. Frog gastric mucosae bathed in chloride-free solutions. Am J Physiol. 1963 Feb;204:233–242. doi: 10.1152/ajplegacy.1963.204.2.233. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- WRIGHT G. H. Net transfers of water, sodium, chloride and hydrogen ions across the gastric mucosa of the rabbit foetus. J Physiol. 1962 Sep;163:281–293. doi: 10.1113/jphysiol.1962.sp006974. [DOI] [PMC free article] [PubMed] [Google Scholar]


