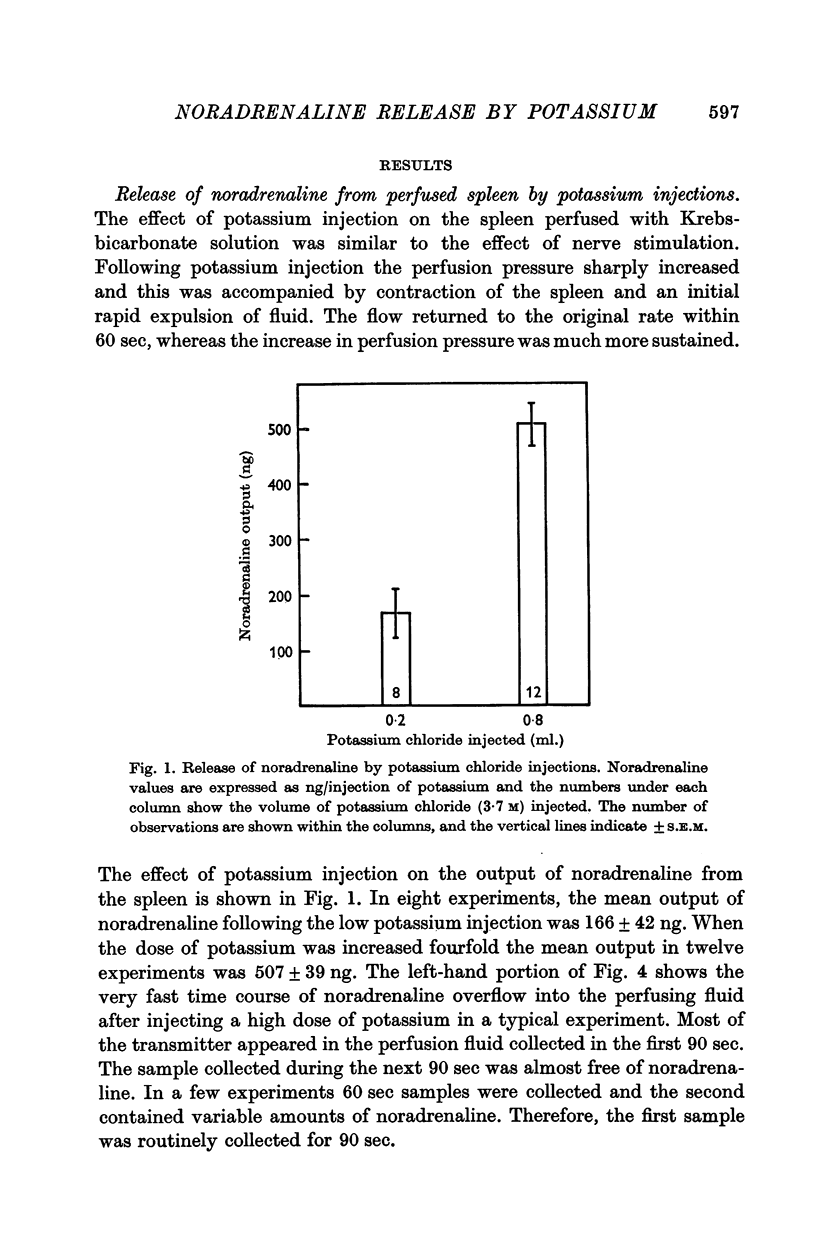
Abstract
1. Cat spleens were perfused with Krebs-bicarbonate solution by means of a constant flow pump. The amount of noradrenaline released by an injection of potassium chloride solution (3·7M) was measured. The dependence of noradrenaline released by KCl on the ionic composition of perfusion medium was determined.
2. In normal cats, the average output was 166 ng following a low dose of KCl (0·2 ml.), and 507 ng following a high dose (0·8 ml.). After phenoxybenzamine treatment, the outputs with both doses were nearly doubled.
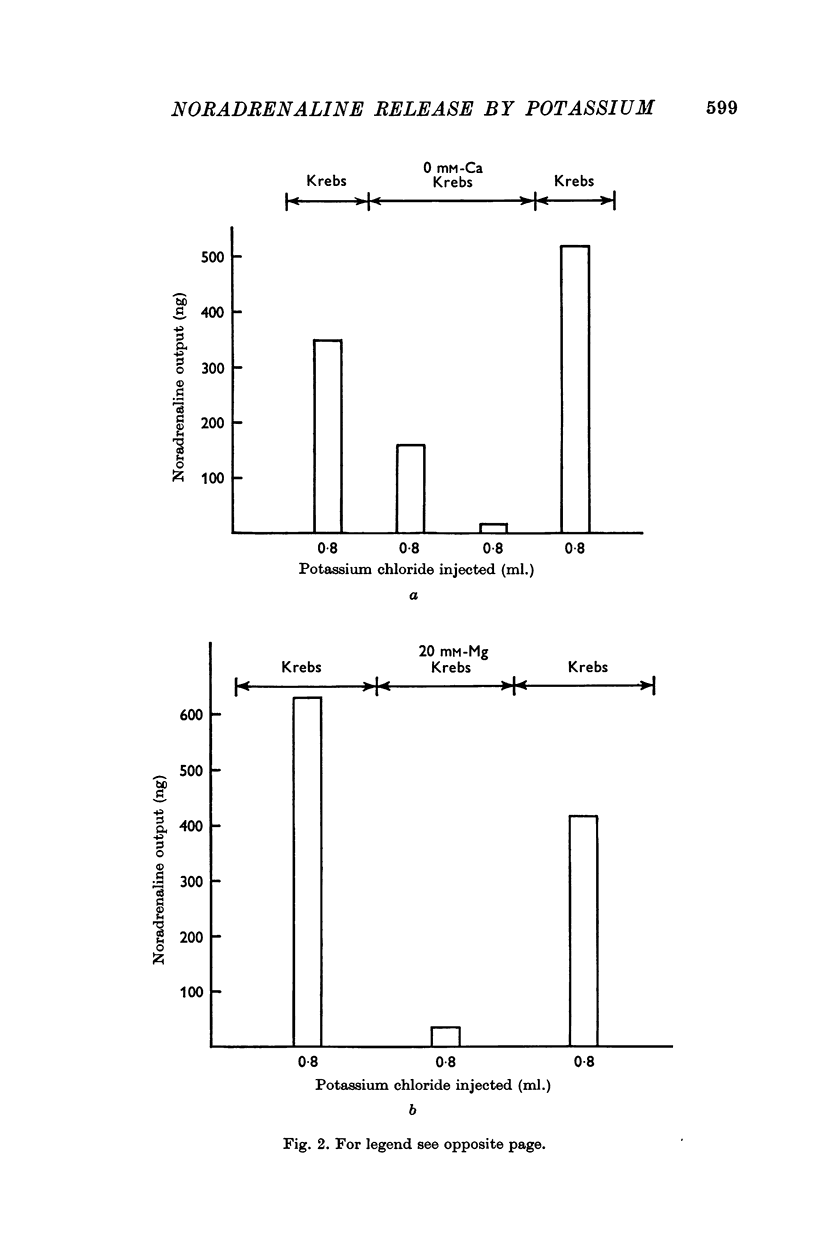
3. In both normal and phenoxybenzamine treated cats, removal of calcium from the perfusing solution nearly abolished the release of noradrenaline. Replacing the calcium in Krebs solution restored the output. Increasing the magnesium concentration to 20 mM also markedly reduced the noradrenaline output.
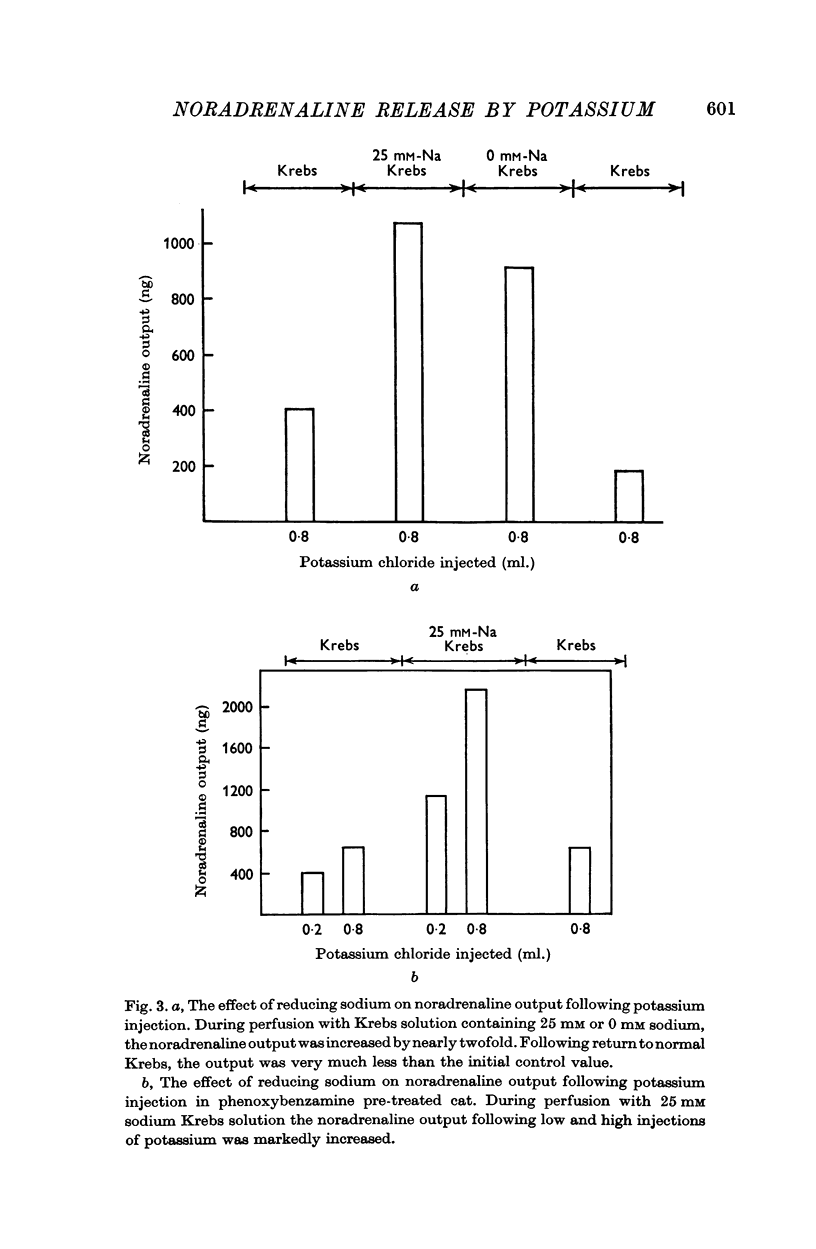
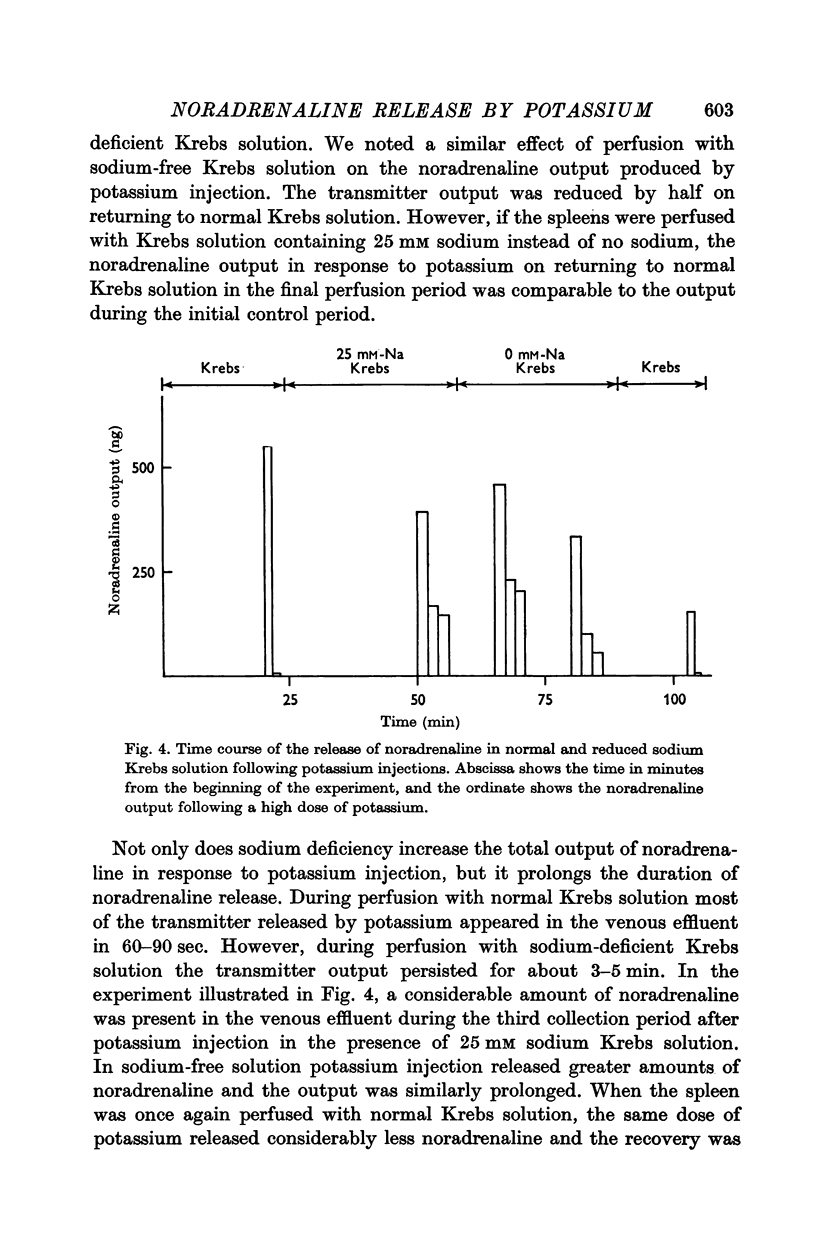
4. Lowering the sodium concentration to either 25 or 0 mM increased the noradrenaline output by nearly two- to threefold. In solutions with reduced concentrations of sodium, noradrenaline was released over longer periods following KCl injections. In phenoxybenzamine treated cats, reduction of sodium in the perfusing solution also increased the noradrenaline output by nearly twofold following either the low or the high dose of KCl.
5. It is concluded that calcium ions are necessary for the release of noradrenaline from post-ganglionic sympathetic nerves following depolarization by potassium ions. It is also suggested that sodium ions are specifically needed for the reincorporation of released transmitter into the sympathetic nerve endings.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The action of sympathomimetic amines in animals treated with reserpine. J Physiol. 1958 Dec 4;144(2):314–336. doi: 10.1113/jphysiol.1958.sp006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F., KIRPEKAR S. M., RIEKER M., SCHWAB A. ACTIONS AND INTERACTIONS OF NOREPINEPHRINE, TYRAMINE AND COCAINE ON AORTIC STRIPS OF RABBIT AND LEFT ATRIA OF GUINEA PIG AND CAT. J Pharmacol Exp Ther. 1963 Oct;142:39–58. [PubMed] [Google Scholar]
- GILLESPIE J. S., KIRPEKAR S. M. THE INACTIVATION OF INFUSED NORADRENALINE BY THE CAT SPLEEN. J Physiol. 1965 Jan;176:205–227. doi: 10.1113/jphysiol.1965.sp007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Kirpekar S. M. The histological localization of noradrenaline in the cat spleen. J Physiol. 1966 Nov;187(1):69–79. doi: 10.1113/jphysiol.1966.sp008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Kirpekar S. M. The uptake and release of radioactive noradrenaline by the splenic nerves of cats. J Physiol. 1966 Nov;187(1):51–68. doi: 10.1113/jphysiol.1966.sp008075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTING G., AXELROD J., WHITBY L. G. Effect of drugs on the uptake and metabolism of H3-norepinephrine. J Pharmacol Exp Ther. 1961 Nov;134:146–153. [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- Harvey A. M., Macintosh F. C. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol. 1940 Jan 15;97(3):408–416. doi: 10.1113/jphysiol.1940.sp003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kravitz E. A. Sodium dependence of transmitter uptake at adrenergic nerve terminals. Mol Pharmacol. 1966 Jul;2(4):360–362. [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Factors influencing noradrenaline uptake by the perfused spleen of the cat. J Physiol. 1968 Feb;194(3):609–626. doi: 10.1113/jphysiol.1968.sp008428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. Movements of Ca in beating ventricles of the frog heart. J Physiol. 1963 Jul;167:551–580. doi: 10.1113/jphysiol.1963.sp007167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITBY L. G., AXELROD J., WEIL-MALHERBE H. The fate of H3-norepinephrine in animals. J Pharmacol Exp Ther. 1961 May;132:193–201. [PubMed] [Google Scholar]


